International Journal of Veterinary Science and Research
Lymphography technique for detecting metastasis in canine malignancies
Anjan Kumar Sahoo*
Cite this as
Sahoo AK (2023) Lymphography technique for detecting metastasis in canine malignancies. Int J Vet Sci Res 9(2): 013-017. DOI: 10.17352/ijvsr.000132Copyright License
© 2023 Sahoo AK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Cancer in animals and human beings spread through both hematogenous and lymphatic routes. While detection of cancer through the hematogenous route is easy delineating the different pathways in lymphatic and lymph nodes involves skill and an advanced imaging system. The present review highlights different techniques for outlining the lymphatic in canine cancer patients. The Sentinel Lymph Node (SLN) is the first or echelon or initial draining node from the primary tumor mass in a regional lymphocentre. Positive (SLN) needs to be evaluated, and surgical planning for its removal along with local tumor control affects the prognosis of any carcinoma or sarcoma. This review discusses different indirect lymphography procedure and their importance to detect the sentinel lymph node in a lymphatic basin both preoperatively and intraoperatively for better surgical assessment of various canine malignancies.
Introduction
Cancer is one of the leading causes of morbidity and mortality in both human beings and companion animals. The occurrence of cancer in canines is twice that of human beings and mainly affects animals above ten years of age and kills 40% - 50% of individuals [1]. The resemblance between canine and human traits has inspired many researchers all over the world to choose a dog as the best preclinical model, which stands higher in the hierarchy than lab animals and lower vertebrates and is an ideal model for in vivo study. Cancer in dogs resembles cancer in man in many ways, such as its clinical manifestation and metastatic potential, pathobiology, including tumor heterogenicity and genomic instability, and pharmacogenomic signature [2]. Thus, Comparative oncology and surgical oncology are two major divisions of surgery that are solely dedicated to the diagnosis and therapeutic management of cancer in laboratory and companion animals.
Cancer in animals occurs in two forms, benign and malignant. When it confines to the Locality, where it develops without showing any systemic signs and symptoms, is referred to as a benign tumor, whereas limitless replicative potential and dissemination of neoplastic cells to distant sites through hematogenous and lymphatic systems give rise to malignant or metastatic cancer. While the majority of sarcomas (cancer of mesenchymal origin) spread through hematogenous invasion, carcinomas (cancer of epithelial origin) and round cell tumors primarily spread through the lymphatic system. Like the blood vascular system, the lymphatic system also has a similar drainage pattern such as small lymphatic capillaries or initial lymphatic (absorb interstitial fluid and cells forming lymph), interstitial space, collecting lymphatic and lymph nodes (structural and functional unit filtering lymph and immune cells) with a unidirectional valve which resist backflow. Lymph nodes are firm, smooth, and ovoid or bean-shaped lymphoid structures with poorly defined cortex and medulla, which act as germinal centers for lymphocytes (small, mature B and T lymphocytes, 85% - 95% or immune cells) and activate a necessary response to a particular threat. Canines have around 60 (sixty) lymph nodes dispersed throughout the body; the sizes of each vary according to age, and condition of the body. Some of the superficial lymph nodes like mandibular, prescapular, superficial inguinal, and popliteal are easily palpable whereas other nodes such as cervical, retropharyngeal, auxiliary, accessory auxiliary, and femoral are generally not palpable. In recent years, various advance diagnostic modalities (radiography, ultrasonography, CT scan, PET-CT, hematobiochemical parameter, histopathology, immunostaining, etc.) have been adopted in canines, for accurately detecting cancer and its metastatic point in tumors of skin and subcutis, soft tissue sarcoma, oral and maxillofacial tumor, perianal tumor, hematopoietic tumor, tumor of skeletal and extra skeletal tissue, tumor of mammary gland tissues, tumor of the reproductive system, and tumor of the urogenital system. Accordingly, appropriate chemotherapy (adjuvant/ neoadjuvant), immunotherapy, radiation therapy [3,4], or a combination of any of these are being attempted as means of therapeutic strategies to increase the survival time of the patient.
Techniques for detecting metastasis in canine malignancies
Tumor staging (determination of the extent of spread of cancer) both in human beings and animals is done for prognostication and planning proper treatment regimens. Staging clearly indicates the malignant nature of the tumor and is based on WHO guidelines for TNM (Tumor, Node, and Metastasis) staging systems [5]. For many years, regional lymph node dissection was routinely accompanied by the surgical procedure even if the nodes appeared clinically normal, which creates unnecessary surgical complications and patient morbidity [6]. In malignant tumors, local recurrence or metastases to regional lymph nodes and distant visceral organs are often observed, resulting in morbidity, shortened survival times, or death of the animal. Therefore, the location of regional lymph nodes and the pattern of lymphatic drainage are critical to the staging and dissection of specific metastatic nodes.
Lymphography (visualizing lymphatic with radio-opaque substance) is one of the several diagnostic techniques to detect lymph node metastasis and predict the expected outcome of the disease process with various therapeutic regimens. Imaging the hemovascular system often requires intravenous administration of contrast agents, while the routes of introducing contrast agents into the lymphatics are through interstitial (intradermal or subcutaneous) administration, direct administration into the lymphatic vessel, or intravenous injection. Visualization of lymphatic pathways can be achieved either through direct lymphography (radiocontrast injection directly into the nodes or lymphatic vessel) or indirect lymphography (injection is made subcutaneously, intramuscularly, or intradermally at adjacent peritumoral structure) [7]. Imaging of contrast uptake and drainage of lymphatic patterns are performed through radiography [8,9], ultrasonography [10], computed Tomodensitometry (CT-IL), or MRI, which helps in the clear identification of contrast-enhanced lymph nodes and lymphatic vessels [11-13]. As the metastatic spread of tumor primarily involves the lymphatic pathway in which lymph nodes or regional nodes play a vital role, so, detecting the first lymph node in the lymphatic basin which drains from the primary tumor, will help in accurately identifying the locoregional metastasis and avoid complete lymphadenectomy in certain patients with further upstaging of tumors. This first lymph node that drains the primary tumor is otherwise called the Sentinel Lymph Node (SLN) and described by Morton, et al. 1992 [14], that a sentinel (= Guard) node is the initial lymph node upon which the primary tumor drains. Otherwise, it is the first-tier node or first-echelon node in the lymphatic basin, which is in the direct drainage pathway from the primary tumor. Therefore, it may not be the nodes located anatomically nearest to particular cancer or nodes in the same location. This also indicates that, if on biopsy and staging, the particular Sentinel lymph node is found negative for cancer, it has not metastasized to the locoregional nodal basin, and the patient is unlikely to have an advanced stage of the disease. Various method of clinical evaluation of lymph node includes palpation, lymph node measurement, fine needle aspiration cytology, imaging with radiography, ultrasound, and computed tomography [15-20]. Use of palpation and cytology alone for evaluation of lymph node metastasis lacks sensitivity and specificity for which concurrent imaging modalities along with administration of dyes, contrast agents, and tracers increase the accuracy of identification on Lymph Node (LN) or sentinel lymph node [15,19-21 ].
Discussion
The lymphatic mapping for sentinel lymph node identification and biopsy in recent years has extended beyond its application from melanoma and breast cancer patients to encompass its implication in several canine malignancies. Detection of the echelon node or initial draining node will have an accurate assessment of the progress of malignant neoplasia and through refinement of pathological examination, (micro) metastasis could be detected within the sentinel node. It will result in a better assessment of the disease at an early stage and lengthen the overall survival time of the clinically ill patient. This happens because, the identification of one node or sometimes more than one, which is rare, avoids patient morbidity in many ways. Usual classical lymphadenectomy involves the dissection of all nodes in a regional lymphocenter which includes both metastatic and non-metastatic nodes resulting in the creation of a larger wound bed, damage to a larger area of soft tissue, and a prolonged period of surgical procedure. The situation becomes more embarrassing and harassing when, after complete regional lymphadenectomy, the tissue sample on examination by a pathologist, is found negative for metastasis or only micrometastasis is detected in one lymph node [22]. On the other hand, it becomes easier for a pathologist to process fewer nodes (usually one to three nodes) and thoroughly analyse for the presence of (micro) metastasis in a sentinel lymph node. It also happens that unnecessary lymphadenectomy eventually causes lymphadenopathy or lymphadenitis of the region drained by the lymph nodes. Hence, with the advancement of knowledge in this area, emphasis is given to the development of a method of detection to accurately identify nodal metastasis in a regional lymphocenter with 100% sensitivity and specificity. The exploration in this area of lymphography originates from the initial lymph node detection method through blue dye and radiocolloid lymphography to today’s radiographic lymphography, CT lymphography, fluorescent lymphography (Indocyanine green -NIR imaging system) [23,24], and ultrasonolymphography to a future prospective analysis through nanocarriers [25,26], photoacoustic imaging [27,28], and magnetic resonance lymphography [29,30]. Among blue dyes, patent blue and its isomer isosulfan blue, both show an allergic and anaphylactic reaction, and concurrently, Methylene Blue (MB) was tested as an alternative tracer for lymphatic mapping and sentinel LN identification. Methylene blue, chemically known as methylthionine hydrochloride is commonly used in many diagnostic and surgical procedures [31]. In lymphoscintigraphy, radiocolloids or radiopharmaceuticals are used which consist of a non-radionuclide portion, which acts as a carrier, and a radionuclide portion which emits a photon that is detected by special imaging equipment like a gamma probe and X-ray and CT scan [32]. Brissot, et al. [33] reported the relevance of SLN mapping through indirect lymphography technique using iodized oil as a radiocontrast agent or marker preoperatively and surgical extirpation of nodes through peritumoral injection of MB. They injected 2 ml – 4 ml of Lipidol UltraFluid TM (Iodized ethyl-esters of the fatty acids of poppy seed oil, Guerbet, France 480 mg Iodine /ml) preoperatively at the peritumoral site into the submucosa or dermis until a “bubble” of IO/MB was seen under the surface of mucosa or skin. Peritumoral injections were done with a 25 G needle into the four quadrants encircling the tumor mass at 0.5 cm - 1 cm distance over 1 - 5 minutes, and imaging was performed with radiography of two orthogonal views. MB was injected following the same principle at exactly the same location 15 minutes before the surgical procedure, and the total volume of injection was 0.5 ml – 1 ml. Dilution was made with dextrose @1:1 for animals weighing below 10 kg. In this study, the rate of identification of SLN with both IO & MB was much higher than the use of individual tracers alone.
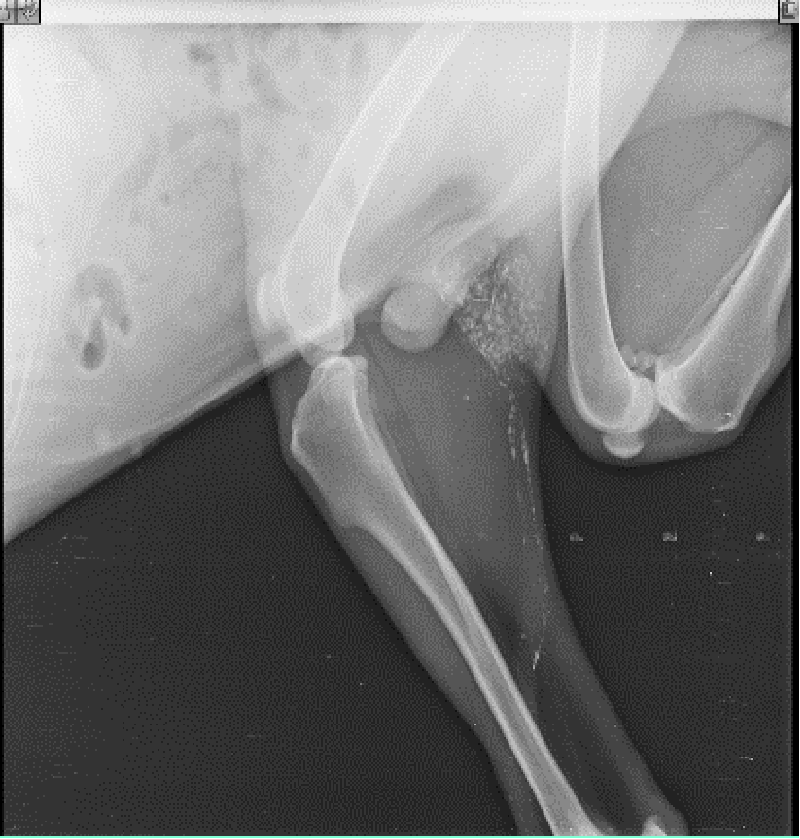
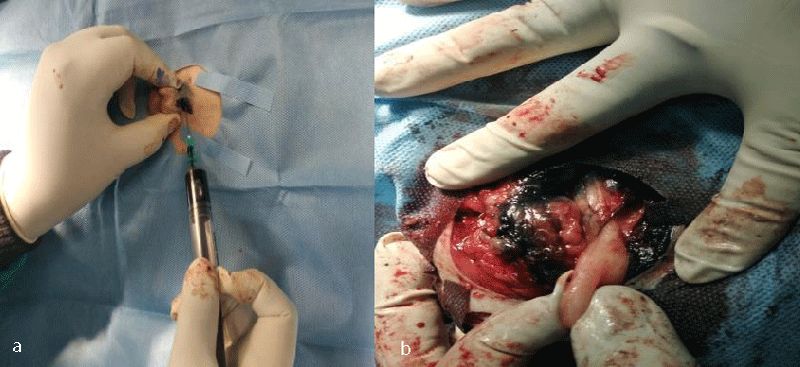
The ultimate aim of all these procedures is to fix a node, upon which the surgeon relies that if any distant spread of malignant cells has occurred, it means that a particular locoregional lymph node harbours the metastasis. The absence of metastasis in the node indicates that all other nodes in the regional lymphocentrum are negative for metastasis and cancer cells are yet to be dispersed through the lymphatic, and the timing is proper for surgical and therapeutic intervention. Herring, et al. [22] removed six lymph nodes in the regional lymhpocentrum, a mandibular lymph node, a medial retropharyngeal lymph node, and a parotid lymph node of both sides, for histopathological analysis. If we conceptualize his ideas of routine removal of all the possible lymphocentrum in a region or area of the body where the need for lymph node assessment or evaluation arises, then there will be unnecessary surgical morbidity, profound surgical stress, and idiopathic lymphadenopathy, which will mask the effect of regional lymphadenectomy. The profound benefits of sentinel lymph node mapping, assessment, and biopsy over blind and indiscriminate lymphadenectomy can also be trailed to human literature. Wong, et al. [34] recommended staging of sentinel lymph node biopsy in newly diagnosed melanoma over Completion Lymph Node Dissection (CLND) in a regional lymphocentrum, as the staging benefits outweigh the risks associated with this procedure and hence CLND is recommended only in case of positive SLN biopsy. Thus, our current research methodology is targeted to the detection of these marked nodes within the regional lymphocentrum, the positiveness of which have a poorer prognosis of survival outcome. Studies like Mayer, et al. [8], and Brissot and Edery [33], generalized the clinical concept to application in all canine malignancies. Collivignarelli, et al. [35], recently reported a thorough clinical investigation of the sentinel lymph node concept in canine mammary carcinoma through indirect radiographic lymphography with Lipidol Ultrafluid. The author in his present research has introspected, analyze, and detailed the result from the use of three commonly used dyes (Iohexol, Lipidol Ultrafluid (Figure 1), and Methylene blue (Figure 2)) in most of the commonly occurring canine malignancies. Since the use of the lymphography technique by Kinmonth [36] several studies have been reported both in the veterinary and human prospective clinical study on the application of both the direct and indirect lymphography procedures with pros and cons. On reviewing the past literature, it was learned that, though direct lymphography has added the advantage of clear opacification of nodes, hot nodes, blue-stained nodes, node enhancement, or fluorescence better in Near-Infrared Light (NIR) with fewer side effects, the procedure involved starting from cannulation into the lymphatic channel to localization of nodes required high skills with invasive procedures which are very painful both for the patient and surgeons, maybe a human being or animals [8]. There are also more chances of seeding or dispersion of malignant cells into the surrounding tissue and risk of pulmonary embolization when the volume of contrast is more than 20 ml [8]. On the other hand, indirect lymphography techniques involving peritumoral, and intraparenchymal injections have a lower risk of tumor capsule damage and subsequent seeding.
Conclusion
In recent decades, as the outpatient record of both human and canine malignancies are exponentially increasing, more concern has grown on non - invasive procedures for accurate diagnosis and staging of both primary tumor and metastatic locoregional lymph node (SLN) so as to deliver more appropriate and effective cancer treatment protocol (chemotherapy, palliative surgery, targeted immunotherapy, radiation therapy, etc.).Therefore, away from conventional lymphography, dyes, and tracers are used as separate or combined for the detection of metastatic points. The combined protocol also helps to detect more than two nodes that drain the primary tumor at the same time.
- Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18(8):781-92. doi: 10.3109/07357900009012210. PMID: 11107448.
- Garden OA, Volk SW, Mason NJ, Perry JA. Companion animals in comparative oncology: One Medicine in action. Vet J. 2018 Oct;240:6-13. doi: 10.1016/j.tvjl.2018.08.008. Epub 2018 Aug 27. PMID: 30268334.
- National Cancer Policy Forum; Board on Health Care Services; Institute of Medicine; National Academies of Sciences, Engineering, and Medicine. The Role of Clinical Studies for Pets with Naturally Occurring Tumors in Translational Cancer Research: Workshop Summary. Washington (DC): National Academies Press (US); 2015 Dec 9. PMID: 26803853.
- Holden SA, Emi Y, Kakeji Y, Northey D, Teicher BA. Host distribution and response to antitumor alkylating agents of EMT-6 tumor cells from subcutaneous tumor implants. Cancer Chemother Pharmacol. 1997;40(1):87-93. doi: 10.1007/s002800050631. PMID: 9137536.
- Owen LN. TNM Classification of Tumors in Domestic Animals, World Heath Organization, 34, Geneva, Switzerland.1980
- Halsted WS. I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg. 1894 Nov;20(5):497-555. doi: 10.1097/00000658-189407000-00075. PMID: 17860107; PMCID: PMC1493925.
- Sheehan R, Hreshchyshyn M, Lin RK, Lessmann FP. The Use of Lymphography as a Diagnostic Method. Radiology. 1961; 76(1):47-53.
- Mayer MN, Silver TI, Lowe CK, Anthony JM. Radiographic lymphangiography in the dog using iodized oil. Vet Comp Oncol. 2013 Jun;11(2):151-61. doi: 10.1111/j.1476-5829.2012.00334.x. Epub 2012 May 26. PMID: 22630597.
- Sahoo AK, Nath I, Senapati SB, Panda SK, Das MR, Patra BK.Apocrine Gland Anal Sac Adenocarcinoma in Dogs: 22 Cases (2015-2020). Indian Journal of Animal Research. 2021. DOI: 10.18805/IJAR.B-4371
- Sahoo AK, Nath I, Senapati SB, Panda SK, Das MR. Patra BK. Comparative Evaluation of Nutraceuticals (Curcuma longa L., Syzygium aromaticum L. and Olea europaea) with Single-agent Carboplatin in the Management of Canine Appendicular Osteosarcoma. Indian Journal of Animal Research. 2021; DOI: 10.18805/IJAR.B-4485.
- Wisner ER, Seibert JA, Katzberg RW. Quantitative methods for indirect CT lymphography. Vet Radiol Ultrasound. 1998 Mar-Apr;39(2):110-6. doi: 10.1111/j.1740-8261.1998.tb01975.x. PMID: 9548137.
- Lurie DM, Seguin B, Schneider PD, Verstraete FJ, Wisner ER. Contrast-assisted ultrasound for sentinel lymph node detection in spontaneously arising canine head and neck tumors. Invest Radiol. 2006 Apr;41(4):415-21. doi: 10.1097/01.rli.0000201230.29925.95. PMID: 16523025.
- Gelb HR, Freeman LJ, Rohleder JJ, Snyder PW. Feasibility of contrast-enhanced ultrasound-guided biopsy of sentinel lymph nodes in dogs. Vet Radiol Ultrasound. 2010 Nov-Dec;51(6):628-33. doi: 10.1111/j.1740-8261.2010.01712.x. PMID: 21158235.
- Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992 Apr;127(4):392-9. doi: 10.1001/archsurg.1992.01420040034005. PMID: 1558490.
- Williams LE, Packer RA. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987-2001). J Am Vet Med Assoc. 2003 May 1;222(9):1234-6. doi: 10.2460/javma.2003.222.1234. PMID: 12725311.
- Ballegeer EA, Adams WM, Dubielzig RR, Paoloni MC, Klauer JM, Keuler NS. Computed tomography characteristics of canine tracheobronchial lymph node metastasis. Vet Radiol Ultrasound. 2010 Jul-Aug;51(4):397-403. doi: 10.1111/j.1740-8261.2010.01675.x. PMID: 20806871.
- Skinner OT, Boston SE, Souza CHM. Patterns of lymph node metastasis identified following bilateral mandibular and medial retropharyngeal lymphadenectomy in 31 dogs with malignancies of the head. Vet Comp Oncol. 2017 Sep;15(3):881-889. doi: 10.1111/vco.12229. Epub 2016 May 16. PMID: 27196324.
- Skinner OT, Boston SE, Giglio RF, Whitley EM, Colee JC, Porter EG. Diagnostic accuracy of contrast-enhanced computed tomography for assessment of mandibular and medial retropharyngeal lymph node metastasis in dogs with oral and nasal cancer. Vet Comp Oncol. 2018 Dec;16(4):562-570. doi: 10.1111/vco.12415. Epub 2018 Jul 10. PMID: 29989306.
- Grimes JA, Matz BM, Christopherson PW, Koehler JW, Cappelle KK, Hlusko KC, Smith A. Agreement Between Cytology and Histopathology for Regional Lymph Node Metastasis in Dogs With Melanocytic Neoplasms. Vet Pathol. 2017 Jul;54(4):579-587. doi: 10.1177/0300985817698209. Epub 2017 Mar 27. PMID: 28346126.
- Fournier Q, Cazzini P, Bavcar S, Pecceu E, Ballber C, Elders R. Investigation of the utility of lymph node fine-needle aspiration cytology for the staging of malignant solid tumors in dogs. Vet Clin Pathol. 2018 Sep;47(3):489-500. doi: 10.1111/vcp.12636. Epub 2018 Jul 16. PMID: 30011068.
- Boston SE, Lu X, Culp WT, Montinaro V, Romanelli G, Dudley RM, Liptak JM, Mestrinho LA, Buracco P. Efficacy of systemic adjuvant therapies administered to dogs after excision of oral malignant melanomas: 151 cases (2001-2012). J Am Vet Med Assoc. 2014 Aug 15;245(4):401-7. doi: 10.2460/javma.245.4.401. PMID: 25075823.
- Herring ES, Smith MM, Robertson JL. Lymph node staging of oral and maxillofacial neoplasms in 31 dogs and cats. J Vet Dent. 2002 Sep;19(3):122-6. doi: 10.1177/089875640201900301. PMID: 12382529.
- Zeng HC, Hu JL, Bai JW, Zhang GJ. Detection of Sentinel Lymph Nodes with Near-Infrared Imaging in Malignancies. Mol Imaging Biol. 2019 Apr;21(2):219-227. doi: 10.1007/s11307-018-1237-4. PMID: 29931432.
- Coufal O, Fait V. Use of indocyanine green and the HyperEye system for detecting sentinel lymph nodes in breast cancer within a population of European patients: a pilot study. World J Surg Oncol. 2016 Dec 1;14(1):299. doi: 10.1186/s12957-016-1060-9. PMID: 27905950; PMCID: PMC5134086.
- Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008 Sep;5(9):763-75. doi: 10.1038/nmeth.1248. PMID: 18756197.
- Polomska AK, Proulx ST. Imaging technology of the lymphatic system. Adv Drug Deliv Rev. 2021 Mar;170:294-311. doi: 10.1016/j.addr.2020.08.013. Epub 2020 Sep 3. PMID: 32891679.
- Steinberg I, Huland DM, Vermesh O, Frostig HE, Tummers WS, Gambhir SS. Photoacoustic clinical imaging. Photoacoustics. 2019 Jun 8;14:77-98. doi: 10.1016/j.pacs.2019.05.001. PMID: 31293884; PMCID: PMC6595011.
- Valluru KS, Wilson KE, Willmann JK. Photoacoustic Imaging in Oncology: Translational Preclinical and Early Clinical Experience. Radiology. 2016 Aug;280(2):332-49. doi: 10.1148/radiol.16151414. PMID: 27429141; PMCID: PMC4976462.
- Suga K, Yuan Y, Ogasawara N, Okada M, Matsunaga N. Localization of breast sentinel lymph nodes by MR lymphography with a conventional gadolinium contrast agent. Preliminary observations in dogs and humans. Acta Radiol. 2003 Jan;44(1):35-42. PMID: 12630996.
- Turkbey B, Hoyt RF Jr, Agarwal HK, Bernardo M, Sankineni S, Johnson L, Grant KB, Rais-Bahrami S, Kobayashi H, Wood BJ, Pinto PA, Griffiths GL, Choyke PL. Magnetic resonance sentinel lymph node imaging of the prostate with gadofosveset trisodium-albumin: preliminary results in a canine model. Acad Radiol. 2015 May;22(5):646-52. doi: 10.1016/j.acra.2014.12.021. Epub 2015 Feb 13. PMID: 25683498; PMCID: PMC4395526.
- Lai HC, Hsu HM, Cherng CH, Lin SL, Wu CT, Yu JC, Yeh CC. Interference of patent blue dye with pulse oximetry readings, methemoglobin measurements, and blue urine in sentinel lymph node mapping: a case report and review of the literature. Acta Anaesthesiol Taiwan. 2011 Dec;49(4):162-4. doi: 10.1016/j.aat.2011.11.004. Epub 2011 Dec 19. PMID: 22221691.
- Povoski SP, Neff RL, Mojzisik CM, O'Malley DM, Hinkle GH, Hall NC, Murrey DA Jr, Knopp MV, Martin EW Jr. A comprehensive overview of radioguided surgery using gamma detection probe technology. World J Surg Oncol. 2009 Jan 27;7:11. doi: 10.1186/1477-7819-7-11. PMID: 19173715; PMCID: PMC2653072.
- Brissot HN, Edery EG. Use of indirect lymphography to identify sentinel lymph node in dogs: a pilot study in 30 tumours. Vet Comp Oncol. 2017 Sep;15(3):740-753. doi: 10.1111/vco.12214. Epub 2016 Feb 22. PMID: 26899244.
- Wong JH, Cagle LA, Morton DL. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg. 1991 Nov;214(5):637-41. doi: 10.1097/00000658-199111000-00015. PMID: 1953118; PMCID: PMC1358621.
- Collivignarelli F, Tamburro R, Aste G, Falerno I, Del Signore F, Simeoni F, Patsikas M, Gianfelici J, Terragni R, Attorri V, Carluccio A, Vignoli M. Lymphatic Drainage Mapping with Indirect Lymphography for Canine Mammary Tumors. Animals (Basel). 2021 Apr 13;11(4):1115. doi: 10.3390/ani11041115. PMID: 33924625; PMCID: PMC8070006.
- KINMONTH JB. Lymphangiography in clinical surgery and particularly in the treatment of lymphoedema. Ann R Coll Surg Engl. 1954 Nov;15(5):300-10. PMID: 13208105.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
