International Journal of Veterinary Science and Research
Epidemiology and diagnostic methods of lumpy skin disease: A Short Review
Chala Guyassa*
Cite this as
Guyassa C (2022) Epidemiology and diagnostic methods of lumpy skin disease: A Short Review. Int J Vet Sci Res 8(2): 064-070. DOI: 10.17352/ijvsr.000115Copyright License
© 2022 Guyassa C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Lumpy skin disease (LSD) is a severe viral disease that is having an impact on the cattle industry. The disease is now widespread in the majority of African countries, and it has lately expanded beyond the continent into the Middle East area. The disease’s symptoms include an initial period of fever, followed by swollen lymph nodes, circumscribed firm skin nodules, and ulcerative lesions. It occurs in all agroclimatic situations, although it is more common in low-lying areas and beside watercourses. It is transmitted by insect vectors among cattle that share comparable pasture and watering sites and gather in the same barn. In this article, the lumpy skin disease virus, its epidemiology, and diagnostic methods are reviewed.
Introduction
Lumpy skin disease is a devastating viral disease that affects cattle and Asian water buffalo and is caused by the lumpy skin disease virus (LSDV). According to the OIE, it is one of the most economically significant viral diseases and is classified as a disease of international concern. The disease is on the OIE’s list of notifiable terrestrial animal diseases because of its great economic importance [1-3]. It causes significant economic losses by decreasing milk production, emaciation, and undergrowth in infected animals, irreparable damage to hides, abortion, infertility, and secondary bacterial infections, which can sometimes result in death [2].
LSDV is a member of the Poxviridae family’s Chordopoxvirinae subfamily and the genus Capripoxvirus (CaPVs). The Poxviridae family is distinguished by its large and complex genome, which is made up of a single, linear molecule of ds DNA that codes for about 200 proteins and is divided into two subfamilies: Chordopoxvirinae, which is responsible for vertebrate poxviruses, and Entomopoxvirinae, which is responsible for insect poxviruses. The genus Capripoxvirus includes viruses including lumpy skin disease virus (LSDV) and sheep and goat poxviruses (SPPV and GTPV) [3,4].
LSDV can only complete its replication cycle in ruminant hosts due to its narrow host range. The disease mostly affects cattle of all ages, both sexes, and breeds, with lactating and pregnant cows being more severely affected [5,6]. However, research suggests that young animals are more vulnerable to the severe form of the disease; Bos Indicus is less susceptible to clinical disease than Bos Taurus, and Asian water buffaloes have also been shown to be susceptible. Despite the fact that LSD has not been detected in goats or sheep, skin lesions have developed in sheep, goats, giraffes, impalas, and Grant’s gazelles housed in proximity to sick cattle [7]. LSDV outbreaks in previously disease-free areas are often linked to the entry of cattle from the affected zone, as well as high temperatures and humidity [8,9].
There has been little research on the diagnostic procedures and epidemiological aspects of LSD, and there is a lack of public knowledge about the disease’s relevance. A detailed investigation of the epidemiological characteristics of LSD and its diagnostic procedures may aid in disease control and prevention [10].
Literature review
History of lumpy skin disease: The first clinical manifestations of LSD were discovered in 1929 in Zambia (now Northern Rhodesia) in the form of skin nodules. It was thought to be either plant poisoning or an allergic reaction to an insect bite at the time [1,2]. Another epidemic of the disease occurred in Botswana in October 1943, and it was named “Ngamiland cattle disease” since it initially appeared in Ngamiland. There was evidence during this period that the disease was caused by an infectious agent [3].
Between 1943 and 1945, the disease was spread to other African countries, including Zimbabwe (Southern Rhodesia) and the Republic of South Africa, where evidence of infectious agent transmission was identified by inoculation of cattle with the suspension of the skin nodules and given the name “Lumpy Skin Disease [4].” The disease spread as a panzootic in South Africa, affecting eight million cattle. During the following decades, LSD progressively spread northwards, and it is now found across the entire African continent except Morocco, Libya, and Algeria [2,3].
In East Africa, LSD was discovered in 1957, 1972, 1974, and 1983 in Kenya, Sudan, West Africa, and Somalia, respectively. Between 1981 and 1983, the disease was seen in Ethiopia’s North Western, Western, and Central regions, with high levels of morbidity and fatality. LSD outbreak was first reported in Egypt in May 1988 [2]. LSD outbreaks have been recorded in Oman in 1984 and 2009. Kuwait reported LSD invasions in 1986 and 1991; Egypt in 1988 and 2006; Israel in 1989 and 2006; Bahrain in 1993 and 2002-2003; Yemen, and the United Arab Emirates in 2000 [5]. In 2013, Turkey reported the first confirmed case of LSD in Europe [11].
Lumpy skin disease virus
Except for dogs, the Poxviridae family comprises the largest viruses capable of spontaneously causing disease in most domestic animals. Chordopoxvirinae, the poxviruses of vertebrates, and Entomopoxvirinae, the poxviruses of insects, are the two subfamilies [5]. Lumpy skin disease virus is a member of the Capripoxvirus genus and the Chordopoxvirinae subfamily. The two additional viral species in this genus are the Sheeppox virus and Goatpox virus (Figure 1). There is only one serotype of LSDV, which is the Neethling virus (a prototype strain of LSDV), and it is closely related antigenically to sheep and goat poxvirus [6].
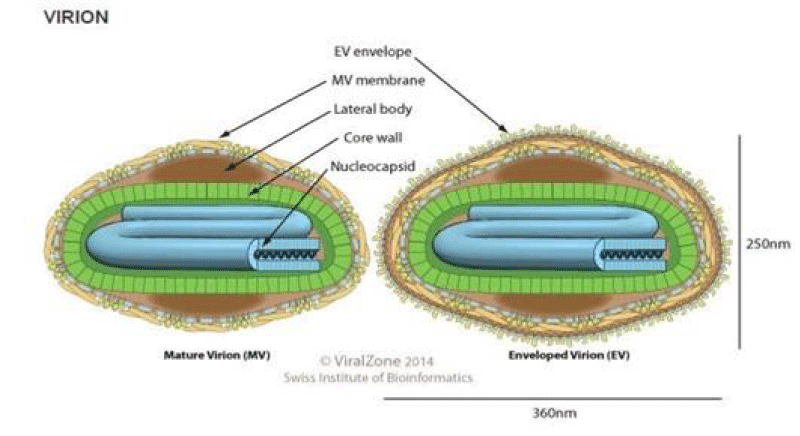
The LSDV virus is a double-stranded DNA virus that measures 300x270x200 nm in size and has a genomic size of approximately 151 kbp with 146 genes. It’s a brick-shaped virus with complicated symmetry [8,13]. Their capsid, or nucleocapsid, is brick or oval-shaped containing the genome and lateral bodies (Figure 2) [6]. Capripoxviruses (CaPVs) are phylogenetically distinct, but they share a high nucleotide sequence identity. Based on the P32 genomic sequence, their phylogenetic analysis showed that members of the genus could be divided into three different clusters: GTPV, SPPV, and LSDV. At the 55th position of P32, sheep poxvirus contains an extra aspartic acid that is absent in GTP and LSD viruses [14].
Lumpy skin disease virus (LSDV) is genetically related to SPPV and GTPV [10]. The genome is conserved and shares 97% of its sequence with viruses from goat pox and sheep pox. Serologic cross-reaction and cross-protection among members account for significant DNA cross-hybridization between species. Cross interactions between poxvirus species are well known [3].
The virus that causes lumpy skin disease is very persistent at ambient temperatures, especially in dried scabs. It can survive in necrotic skin nodules for up to 33 days or longer, in dehydrated crusts for up to 35 days, and in air-dried hides for at least 18 days. Sensitivity to heat differs among strains [16] Figure 3.
Epidemiology of lumpy skin disease
Occurrence of the disease: LSD is prevalent in most African countries, notably in the Sub-Saharan area [5,17]. It extended to South-East Europe, the Balkans, the Caucasus, Russia, and Kazakhstan after 2012 [7]. Field outbreaks may be severe and widespread infections with high rates of morbidity and mortality, while others may have few affected animals and few or no reported deaths. But in general, outbreaks are more severe when the infection is first introduced to a region and then decreased, most likely due to the development of widespread immunity. Morbidity rates during epizootics approach 80%, but are closer to 20% in endemic areas [9].
Hosts and susceptibility
LSDV is highly host-specific (domestic cattle and water buffaloes) with the exception that some strains may replicate in sheep and goats. However, no epidemiological investigations have found small ruminants to be viral reservoirs as of yet [5,11]. Domestic cattle and Asian water buffalo are the most vulnerable animals during LSD outbreaks in the field [5,18]. Some wild animals, such as the giraffe (Giraffe Camelopardalis) and the impala (Aepyceros melampus), are also vulnerable to experimental infection, but their role is unknown [19]. Rather than the virus’s virulence, the host animal’s susceptibility is determined by its immunological state, age, and breed [20].
Sources of the virus
Nodules that occur on the mucous membranes of the eyes, nose, mouth, rectum, udder, and genitalia also ulcerate and release enough viruses which can serve as sources of the virus. Approximately half of the infected animals may develop clinical signs; the majority of experimentally infected animals will become viremic and a source of the virus. LSD virus was found in saliva for 11 days, semen for 22 days, and skin nodules for 33 days in experimentally infected cattle, but not in urine or faces [2]. Because Capripoxviruses are very resistant to physical and chemical conditions, they may survive in lesions or scabs for extended periods of time and have a great affinity for dermal tissues [21].
Transmission
In most of Sub-Saharan Africa, LSD has been seen to occur after seasonal rains, when the number of certain arthropod species increases [21]. The study that looked at the risk variables involved with the development of LSD in Ethiopia discovered that a warm and humid agro-climate, which supports an abundance of vector population, was linked to a higher incidence of LSD [22]. LSDV can be mechanically transmitted by a number of hematophagous arthropod vectors, according to evidence from several sources. The disease is high, with 50-60% attack rates where mosquito populations are abundant and low, 5-15% morbidity in arid areas where there are fewer potential mechanical vectors [2,23].
Mechanical transmission of some poxvirus species by insect vectors such as Stomoxys calcitrans may occur due to high viral loads in skin lesions [24]. Invasive blood-feeding arthropods, such as mosquitoes and sand flies, are suspected to be associated with LSD outbreaks characterized by generalized lesions [25]. Stomoxys calcitrans and Biomyia fasciata were caught after being fed on sick cows, and the LSD virus was isolated from them [26]. Chihota et al found that Aedes aegypti female mosquitos can mechanically transmit LSDV from infected cattle to susceptible cattle [27]. Such a vector feeding regularly and changing hosts between feedings is likely to transmit LSDV mechanically [26]. Chihota et al identified the LSDV genome in mosquitoes (Anopheles stephensis and Culex quinquefasciatus) and biting midges (Culicoides nubeculosus) feeding on LSD-positive animals, but did not observe LSDV transmission by these insects.
Direct and indirect contact might be minor sources of infection (e.g., through infective saliva contaminated feed and water). Poxviruses are extremely resistant and can survive in infected tissue for more than 120 days, or longer. The virus is also identified in blood, nasal discharge, lachrymal secretion, semen, and saliva, which are thought to be the primary routes of LSD transmission [28]. Because the LSD virus can survive for long periods of time in both milk and semen, other potential transmission vectors include nursing cow milk and infected bull semen [29].
Clinical signs and pathogenesis
Large skin nodules covering all regions of the body, fever, swollen lymph nodes, lack of appetite, decrease in milk production, depression and unwillingness to move, nasal discharge, and lachrymation are all symptoms of the disease. In the field, it has a 2 to 4-week incubation period [2]. The nodules that grow on the skin range in size from 2 to 7 cm in diameter, appearing as well-circumscribed regions of erect hair, round, firm, and slightly raised from the surrounding skin, and they are especially noticeable in short-haired animals [30].
During the acute stage of skin lesions, histopathological changes such as vasculitis and lymphangitis with subsequent thrombosis and infarction, which result in edema and necrosis are seen. Serum may have leaked at first, followed by a distinctive inverted greyish pink conical zone of necrosis from LSD skin nodules. Congestion, hemorrhages, and edema are present in adjacent tissue. Secondary bacterial infections are prevalent in necrotic cores, as are enlarged lymph nodes [2].
Diagnosis of lumpy skin disease
LSD is frequently diagnosed in the field based on the disease’s typical clinical characteristics. LSD should be considered clinically when there are distinctive skin nodules, fever, and enlargement of superficial lymph nodes. Thus, the differential diagnosis of LSD is mainly based on distinctive clinical indications. Milder and subclinical forms, on the other hand, need fast and dependable laboratory testing to confirm the diagnosis [31]. Detecting viral DNA using conventional or real-time polymerase chain reaction (PCR) is the most often utilized way of diagnosing LSD. Other different molecular assays are also favored diagnostic methods or serology-based diagnostic tests that identify antibodies to the LSD virus [3,7,11].
Virus isolation
Virus isolation is critical in the confirmation of clinical disease and determination of the isolate. This is the method used in the samples to test the virus’s viability [11]. To propagate LSDV, a number of primary cells or cell lines of bovine, ovine, or caprine origin are utilized. The virus may also grow on the chorioallantoic membrane of embryonated chicken eggs and African green monkey kidney (Vero) cells [7]. It grows slowly in cell cultures, and the cytopathic effect (CPE) is generally detectable five to seven days after inoculation [16,32]. LSDV induces a specific cytopathic effect (CPE) and intracytoplasmic inclusion bodies in cell culture, which differs from infection with Bovine herpesvirus 2, which causes pseudo-lumpy skin disease and causes syncytia and intranuclear inclusion bodies in cell culture [3,7].
Molecular detection methods
Molecular diagnostic testing is critical for monitoring the spread of these viruses and controlling disease outbreaks. LSD virus confirmation in the laboratory may be done quickly utilizing a Capripoxvirus-specific PCR approach or by demonstrating characteristic Capripoxvirions in biopsy material or dried crusts using transmission electron microscopy (TEM). The genome has been detected utilizing Capripoxvirus-specific primers for the attachment protein and fusion protein genes, and multiple conventional and real-time PCR technologies have been developed for use on blood, tissue, and sperm materials [3,14,33].
For Capripoxvirus, the real-time PCR approach using primers and a probe was verified [2,34]. Molecular assays employing loop-mediated isothermal amplification to identify Capripoxvirus genomes have been shown to have sensitivity and specificity comparable to real-time PCR, with a simpler approach and a cheaper cost [35,36].
Serological tests
Serological assays for LSDV include the indirect fluorescent antibody test (IFAT), viral neutralization, enzyme-linked immunosorbent assays (ELISA), and immunological blotting (Western blotting) [33]. The only serologically approved test available is the Virus neutralization test (VNT). Neutralizing antibodies occur 3-4 days after the onset of clinical symptoms and reach maximal titer levels in 2-3 weeks. The agar gel immune diffusion test (AGID) and IFAT are less specific than VNT due to cross-reactivity with antibodies to other poxviruses. Western blotting is sensitive and specific, but it is difficult and expensive to perform. Some ELISAs for antibody detection have been identified, but none have been verified sufficiently to advise for use [7,21].
Differential diagnosis
The main differential diagnosis is pseudo-LSD induced by bovine herpesvirus 2 (BoHV2). Pseudo-lumpy skin disease (caused by herpes virus-2) cutaneous lesions involve only the epidermis and produce a scab after sloughing; systemic symptoms do not occur. This is usually a milder clinical disease with superficial nodules that resemble only the early stages of LSD. Histopathological features of BoHV-2 infection that are not found in LSD include intra-nuclear inclusion bodies and viral syncytia [7,9].
Other differential diagnoses include photosensitization, dermatophilosis, dermatophytosis, bovine farcy, actinobacilosis, actinomycosis, urticaria, insect bites, nocardiasis, besnoitiosis, demodicosis, onchocerciasis, cowpox, and pseudo-cowpox (for integumentary lesions). Bluetongue, foot and mouth illness, malignant catarrhal fever, bovine viral diarrhea, bovine popular stomatitis, and infectious bovine rhinotracheitis are all possible diagnoses for mucosal lesions [14,37].
Economic importance
Capripoxviruses are growing as a global threat to sheep, goats, and cattle [21]. Lumpy skin disease causes significant economic losses due to decreased feed intake, milk production, weight conversion, abortion and infertility, damaged hides, temporary or permanent infertility in males and females, mastitis, and mortality rates of up to 40%, even as mortality rates rarely exceed 3%. Furthermore, the disease is a major notifiable disease that impedes international trade [2,12,21,38].
The disease’s economic impact was mostly owing to its high morbidity rate rather than its fatality rate [38]. As a result, the financial impact of these losses on herd owners, consumers, and industries that produce animal goods and byproducts is significant [22,30].
Control and prevention
LSD treatment is only symptomatic, with antimicrobial therapy used to prevent subsequent bacterial infections [39]. Because movement restrictions and the removal of affected animals are typically ineffective, vaccination is the only practical and economically viable strategy for controlling the spread of the disease and improving cattle productivity in endemic areas [7,11,33]. Vaccinating animals every year might keep LSD under control [40].
Inactivated vaccines are less effective, so several live attenuated vaccines have been developed and used across the world. These vaccines are inexpensive and give enough protection provided sufficient herd immunity (above 80%) is maintained by yearly immunizations [41]. Four live attenuated CaPV strains have been employed as vaccines for the control of LSD in endemic regions, helping to reduce losses from lumpy skin disease [13,14]. These are: a strain of the Kenyan sheep and goat pox virus; the Yugoslavian RM 65 sheep pox strain; the Romanian sheep pox strain; and a lumpy skin disease virus strain from South Africa [5].
Animals that have recovered from infection with any of the Capripoxvirus strains studied so far, whether bovine, ovine, or caprine, share a major neutralizing site and are resistant to infection with any other strain. Immunity against poxviruses is both humoral and cell-mediated [42]. Cattle can be protected against LSD by employing Capripoxvirus strains originating from sheep or goats, such as the Romanian sheep pox strain utilized in Egypt. 14 Strict quarantines and the avoidance of the introduction of infected animals into healthy herds, isolation, and prohibition of animal movements, slaughtering of all sick and infected animals (depopulation of infected and exposed animals), proper disposal of carcasses (incineration), cleaning and disinfection of the premises, and insect control can all help to control an outbreak [2,36] Table 1.
Conclusion and recommendation
Lumpy skin disease is one of the most economically significant transboundary, viral diseases of domestic cattle. It is economically significant in animals because of chronic debility, decreased milk production and weight, damaged skins, abortion, and mortality [2]. LSD is currently present in the majority of African and Middle Eastern countries. LSD is often diagnosed based on specific clinical signs and differential diagnoses. Milder and subclinical forms, on the other hand, require quick and accurate laboratory testing to prove the diagnosis [31]. The disease’s economic impact was mostly due to its high morbidity rate rather than its mortality rate [38].
Based upon the above conclusion, the following recommendations are forwarded:
- The disease's global expansion requires special attention.
- Action plans for effective control and prevention should be developed to reduce the disease's economic losses.
- If LSD is introduced into a disease-free country, rapid identification and culling of infected herds, as well as ring vaccination, should be undertaken.
- Additional research into control strategies is required.
I’d like to thank the Animal Health Institute for allowing me to use various sources and the internet to write this article.
- Bagla VP The demonstration of lumpy skin disease virus in semen of experimentally infected bulls using different diagnostic techniques (Doctoral dissertation, University of Pretoria). 2008.
- Tuppurainen ES, Oura CA. Review: lumpy skin disease: an emerging threat to Europe, the Middle East and Asia. Transbound Emerg Dis. 2012 Feb;59(1):40-8. doi: 10.1111/j.1865-1682.2011.01242.x. Epub 2011 Jul 12. PMID: 21749675.
- Abdulqa HY, Rahman HS, Dyary HO, Othman HH. Lumpy skin disease. Am J Reprod Immunol. 2016; 1: 2476-1974.
- Swiswa S, Masocha M, Pfukenyi DM, Dhliwayo S, Chikerema SM. Long-term changes in the spatial distribution of lumpy skin disease hotspots in Zimbabwe. Trop Anim Health Prod. 2017 Jan;49(1):195-199. doi: 10.1007/s11250-016-1180-9. Epub 2016 Oct 26. PMID: 27785763.
- Al-Salihi K. Lumpy skin disease: Review of literature. MRVSA. 2014; 3: 6-23
- Gumbe AAF. Review on lumpy skin disease and its economic impacts in Ethiopia. J Dairy Vet Anim Res. 2018; 7: 39-46.
- OIE. Terristerial manual chapter 2.4.13. Lumpy Skin Disease. 2017;
- Shakya S.. Identification and Molecular Characterization Of Immunogenic Proteins Of Capripox Virus (Doctoral dissertation, Govind Ballabh Pant University of Agriculture and Technology; Pantnagar). 2001.
- Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Veterinary Medicine E-Book: A textbook of the diseases of cattle, horses, sheep, pigs and goats. Elsevier Health Sciences. Edition 10th. Sounders Elsevier. 2006.
- Gari G, Bonnet P, Roger F, Waret-Szkuta A. Epidemiological aspects and financial impact of lumpy skin disease in Ethiopia. Prev Vet Med. 2011 Dec 15;102(4):274-83. doi: 10.1016/j.prevetmed.2011.07.003. Epub 2011 Aug 17. PMID: 21852008.
- Tuppurainen E. Diagnostic assays for the detection of lumpy skin disease virus and antibodies. EMPRES. 2017; 47: 7-9.
- EFSA Panel on Animal Health and Welfare (AHAW). Scientific Opinion on lumpy skin disease. EFSA Journal. 2015. 13: 3986.
- Tulman ER, Afonso CL, Lu Z, Zsak L, Sur JH, Sandybaev NT, Kerembekova UZ, Zaitsev VL, Kutish GF, Rock DL. The genomes of sheeppox and goatpox viruses. J Virol. 2002 Jun;76(12):6054-61. doi: 10.1128/jvi.76.12.6054-6061.2002. PMID: 12021338; PMCID: PMC136203.
- Hosamani M, Mondal B, Tembhurne PA, Bandyopadhyay SK, Singh RK, Rasool TJ. Differentiation of sheep pox and goat poxviruses by sequence analysis and PCR-RFLP of P32 gene. Virus Genes. 2004 Aug;29(1):73-80. doi: 10.1023/B:VIRU.0000032790.16751.13. PMID: 15215685.
- Gelaye E, Belay A, Ayelet G, Jenberie S, Yami M, et al. Capripox disease in Ethiopia: Genetic differences between field isolates and vaccine strain, and implications for vaccination failure. Antivir Res. 2015; 119: 28-35.
- Viral zone. Capripoxvirus. 2014.
- Rao TV, Bandyopadhyay SK. A comprehensive review of goat pox and sheep pox and their diagnosis. Anim Health Res Rev. 2000 Dec;1(2):127-36. doi: 10.1017/s1466252300000116. PMID: 11708598.
- Brenner J, Bellaiche M, Gross E, Elad D, Oved Z, Haimovitz M, Wasserman A, Friedgut O, Stram Y, Bumbarov V, Yadin H. Appearance of skin lesions in cattle populations vaccinated against lumpy skin disease: statutory challenge. Vaccine. 2009 Mar 4;27(10):1500-3. doi: 10.1016/j.vaccine.2009.01.020. Epub 2009 Jan 30. PMID: 19186204.
- El-Nahas EM, El-Habbaa AS, El-Bagoury GF, Radwan MEI. Isolation and identification of lumpy skin disease virus from naturally infected buffaloes at Kaluobia, Egypt. Glob Vet. 2011; 7: 234-237
- Padilla LR, Dutton CJ, Bauman J, Duncan M. XY male pseudohermaphroditism in a captive Arabian oryx (Oryx leucoryx). J Zoo Wildl Med. 2005 Sep;36(3):498-503. doi: 10.1638/04-006.1. PMID: 17312771.
- Tageldin MH, Wallace DB, Gerdes GH, Putterill JF, Greyling RR, Phosiwa MN, Al Busaidy RM, Al Ismaaily SI. Lumpy skin disease of cattle: an emerging problem in the Sultanate of Oman. Trop Anim Health Prod. 2014 Jan;46(1):241-6. doi: 10.1007/s11250-013-0483-3. Epub 2013 Oct 7. PMID: 24097247; PMCID: PMC3895213.
- Babiuk S, Bowden TR, Boyle DB, Wallace DB, Kitching RP. Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transbound Emerg Dis. 2008 Sep;55(7):263-72. doi: 10.1111/j.1865-1682.2008.01043.x. PMID: 18774991.
- Gari G, Waret-Szkuta A, Grosbois V, Jacquiet P, Roger F. Risk factors associated with observed clinical lumpy skin disease in Ethiopia. Epidemiol Infect. 2010 Nov;138(11):1657-66. doi: 10.1017/S0950268810000506. Epub 2010 Mar 17. PMID: 20233495.
- Kitching RP, Mellor PS. Insect transmission of Capripoxvirus. Res Vet Sci. 1986 Mar;40(2):255-8. PMID: 3010413.
- Weiss KE. Lumpy skin disease virus. Virol Monogr. 1968; 3:111-131.
- Chihota CM, Rennie LF, Kitching RP, Mellor PS. Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae). Epidemiol Infect. 2001 Apr;126(2):317-21. doi: 10.1017/s0950268801005179. PMID: 11349983; PMCID: PMC2869697.
- Chihota CM, Rennie LF, Kitching RP, Mellor PS. Attempted mechanical transmission of lumpy skin disease virus by biting insects. Med Vet Entomol. 2003 Sep;17(3):294-300. doi: 10.1046/j.1365-2915.2003.00445.x. PMID: 12941014.
- Honhold N, Douglas I, Geering W, Shimshoni A, Lubroth J. Good emergency management practice: the essentials. FAO Animal Production and Health Manual. 2011;
- Irons PC, Tuppurainen ES, Venter EH. Excretion of lumpy skin disease virus in bull semen. Theriogenology. 2005 Mar 15;63(5):1290-7. doi: 10.1016/j.theriogenology.2004.06.013. PMID: 15725437.
- Alemayehu G, Zewde G, Admassu B. Risk assessments of lumpy skin diseases in Borena bull market chain and its implication for livelihoods and international trade. Trop Anim Health Prod. 2013 Jun;45(5):1153-9. doi: 10.1007/s11250-012-0340-9. Epub 2012 Dec 29. PMID: 23274626; PMCID: PMC3661036.
- El-Kholy AA, Soliman HM, Abdelrahman KA. Polymerase chain reaction for rapid diagnosis of a recent lumpy skin disease virus incursion to Egypt. Arab J Biotechnol. 2008; 11: 293-302.
- Binepal YS, Ongadi FA, Chepkwony JC. Alternative cell lines for the propagation of lumpy skin disease virus. Onderstepoort J Vet Res. 2001 Jun;68(2):151-3. PMID: 11585094.
- Abera Z, Degefu H, Gari G, Ayana Z. Review on Epidemiology and Economic Importance of Lumpy Skin Disease. Int J Appl Virol. 2015a 4: 8-12.
- Bowden TR, Coupar BE, Babiuk SL, White JR, Boyd V, Duch CJ, Shiell BJ, Ueda N, Parkyn GR, Copps JS, Boyle DB. Detection of antibodies specific for sheeppox and goatpox viruses using recombinant Capripoxvirus antigens in an indirect enzyme-linked immunosorbent assay. J Virol Methods. 2009 Oct;161(1):19-29. doi: 10.1016/j.jviromet.2009.04.031. Epub 2009 May 6. PMID: 19426763.
- Das A, Babiuk S, McIntosh MT. Development of a loop-mediated isothermal amplification assay for rapid detection of Capripoxviruses. J Clin Microbiol. 2012 May;50(5):1613-20. doi: 10.1128/JCM.06796-11. Epub 2012 Feb 22. Erratum in: J Clin Microbiol. 2013 Sep;51(9):3164. PMID: 22357504; PMCID: PMC3347125.
- Murray L, Edwards L, Tuppurainen ES, Bachanek-Bankowska K, Oura CA, Mioulet V, King DP. Detection of Capripoxvirus DNA using a novel loop-mediated isothermal amplification assay. BMC Vet Res. 2013 May 1;9:90. doi: 10.1186/1746-6148-9-90. PMID: 23634704; PMCID: PMC3649941.
- Abera Z, Degefu H, Gari G. Assessment of Distribution and Associated Risk Factors of Lumpy Skin Disease in Selected Districts of West Wollega Zone, Western Ethiopia. Ac J Ani Dis. 2015b 4: 130-140.
- Abutarbush SM, Ababneh MM, Al Zoubi IG, Al Sheyab OM, Al Zoubi MG, Alekish MO, Al Gharabat RJ. Lumpy Skin Disease in Jordan: Disease Emergence, Clinical Signs, Complications and Preliminary-associated Economic Losses. Transbound Emerg Dis. 2015 Oct;62(5):549-54. doi: 10.1111/tbed.12177. Epub 2013 Oct 21. PMID: 24148185.
- Molla W, de Jong MCM, Frankena K. Temporal and spatial distribution of lumpy skin disease outbreaks in Ethiopia in the period 2000 to 2015. BMC Vet Res. 2017 Nov 6;13(1):310. doi: 10.1186/s12917-017-1247-5. PMID: 29110713; PMCID: PMC5674741.
- Thomas L. Lumpy-skin disease, a disease of socioeconomic importance. J Med Virol. 2002; 76: 6054-6061.
- Boumart Z, Daouam S, Belkourati I, Rafi L, Tuppurainen E, Tadlaoui KO, El Harrak M. Comparative innocuity and efficacy of live and inactivated sheeppox vaccines. BMC Vet Res. 2016 Jun 29;12(1):133. doi: 10.1186/s12917-016-0754-0. PMID: 27357388; PMCID: PMC4928353.
- Tuppurainen E, Dietze K, Wolff J, Bergmann H, Beltran-Alcrudo D, Fahrion A, Lamien CE, Busch F, Sauter-Louis C, Conraths FJ, De Clercq K, Hoffmann B, Knauf S. Review: Vaccines and Vaccination against Lumpy Skin Disease. Vaccines (Basel). 2021 Oct 6;9(10):1136. doi: 10.3390/vaccines9101136. PMID: 34696244; PMCID: PMC8539040.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
