International Journal of Veterinary Science and Research
Acremonium species skin infection in a female French bulldog
Vladislav E Sobolev*
Cite this as
Sobolev VE (2022) Acremonium species skin infection in a female French bulldog. Int J Vet Sci Res 8(2): 046-049. DOI: 10.17352/ijvsr.000112Copyright License
© 2022 Sobolev VE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Currently, the role of opportunistic pathogenic microflora in the total number of dermatological patients in veterinary clinics has increased significantly. As the author’s personal experience shows, the clinical significance of fungal opportunistic microflora is often underestimated by veterinary professionals. The article considers a case of successful treatment of Acremonium spp skin infection in an 11-year-old female French bulldog. A course of therapy using itraconazole, terbinafine, probiotics, and topical treatment of the skin with an aerosol product containing 8-oxyquinoline (C9H7NO) stopped the infection within 8 weeks. A follow-up examination of the dog after 3 months showed 90% hair regrowth and no evidence of infection.
Introduction
Opportunistic fungi Acremonium spp are quite widely distributed in the environment. They are found in soil, ponds, ground parts of plants, and in association with mold fungi. As a potential pathogen, fungi of this genus have been known in human medicine for quite some time. Cases of human eye infections by Acremonium sp. fungi were described as early as the 1970s [1]. Currently, 10 representatives of fungi of this genus capable of causing infections in invertebrates have been identified. The most pathogenic representative of the class is A. Kiliense. Incompleteness or damage to the defense mechanisms of the immune system contributes to the emergence of infection.
Case report
Anamnesis
An 11-year-old French bulldog, a neutered female, was admitted to the clinic in the summer with signs of localized alopecia, accompanied by severe itching, scratching, and anxiety. The owner said that the dog had been swimming in a lake in a city park in St. Petersburg for 7-12 days before clinical signs of infection appeared. The owner attributed the dog’s skin lesions to the polluted and environmentally unfriendly water in the lake.
Clinical examination and laboratory results
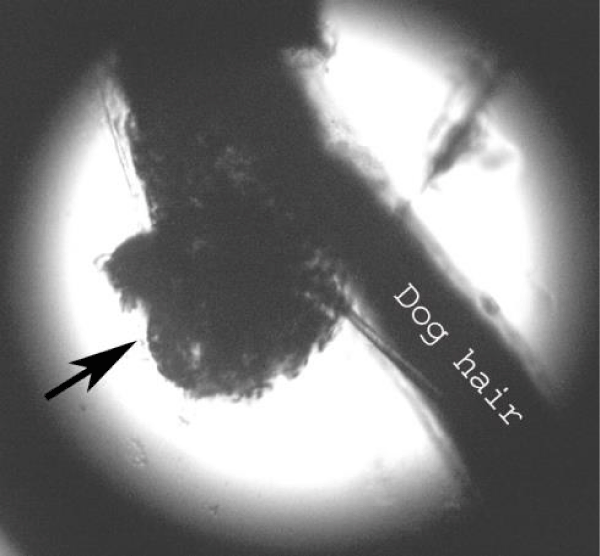
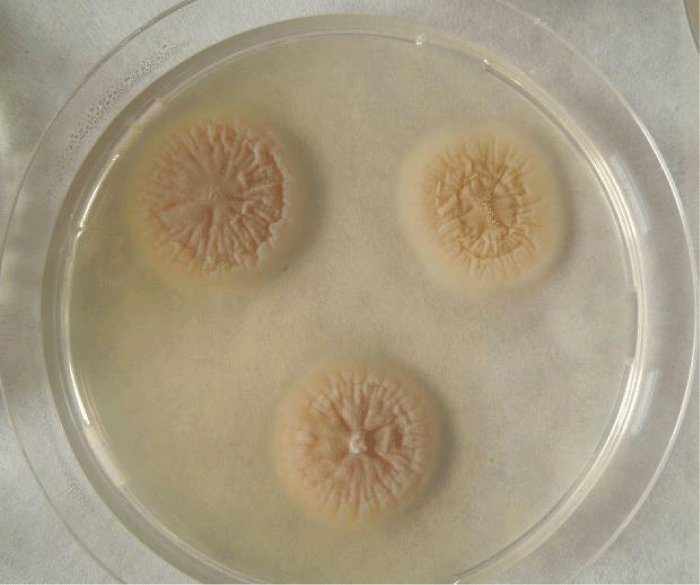
Examination of the skin revealed multiple areas of alopecia, measuring 3-5 cm, localized predominantly on the back and sacrum. The skin in these areas is thickened and moderately oedematous, and the local temperature is elevated. Dry, greyish-white scales appear on the surface of the bald patches. Mechanical removal of the scales will increase the dog’s itching and restlessness. The hair in the damaged areas of the skin is poorly retained and falls out, resulting in multiple pockets of alopecia after 3-5 days from the first signs of the disease (Figure 1). The initial step in diagnosis was a microscopic examination of an undyed preparation of hair and skin scales. For better detailing of the structure, the hair was treated with a 10% sodium bicarbonate solution for 10-15 minutes, followed by light microscopy under low (x80) and medium magnification (x200). The infection damaged the surface of the hair predominantly in the ectothrix type (Figure 2). Acremonium spp fungi were isolated and identified by mycological examination (culture on Sabouraud’s agar) at the Research Institute of Medical Mycology (St. Petersburg ) Figures 3-5.
Therapy and follow up
Initially, itraconazole at a dose of 15 mg/kg body weight, twice daily for 2 weeks, was given as a systemic antifungal agent. Daily in a mixture with food the dog received Vetom 1.1 (Research Center©, Russia) containing a dry culture of probiotic microorganisms Bacillus subtilis in a dose of 50 mg/kg of body weight 2 times a day. Additionally, once every 3-5 days the skin was treated with a shampoo containing 2% ketaconazole.
After 2 weeks of treatment, the dog was evaluated and the owner was interviewed to objectify the symptoms. A reduction in the intensity of itching reactions and a suspension of further alopecia progression was noted. However, the dog continued to experience itching and anxiety. The results of the therapy were therefore found to be unsatisfactory. Terbinafine (Lamisil) at a dose of 10 mg/kg body weight 2 times daily after meals for 3 weeks was used to continue the course of systemic antifungal therapy. Topical skin treatments included washing with Doctor veterinary shampoo (Goodman©, Russia) containing birch tar at intervals of once every 5 days.
After 2 weeks, the effectiveness of the prescribed treatment was re-evaluated. There was an improvement in the dog’s skin condition. No new areas of skin damage were detected, but the dog was still affected by itching. Given the long course of systemic therapy, it was decided to shift the focus of treatment to topical and probiotic therapy. For topical treatment, the aerosol preparation Bidezmik (Bionit©, Russia) containing 8-oxyquinoline was used. The preparation was sprayed on the affected skin areas twice a day for 7 days and then once a day, for 3 weeks. To correct immunity, probiotic preparations Vetom 1.1 in the same dose and Multibacterin Omega-10 (Russia) containing live symbiont culture of lactic acid bacteria Lactobacillus acidophilus (109 CFU in 1 ml) were used in a mixture with feed.
Treatment efficacy was assessed after 4 weeks. The owner of the dog noted the complete disappearance of itching and anxiety. New hair growth was observed in the areas of hair loss. Mycological testing was negative. Further follow-up of the animal for 3 months showed no recurrence of the infection and the hair was 90% recovered at the last follow-up.
Discussion
Acremonium spp fungi are ubiquitous in the environment and are found in soil, plant debris, and decaying fungi, with A. Kiliense (Sarocladium kiliense) is the most important of the 10 species reported as an infectious etiology in vertebrates [2]. In an immunocompetent host, a mycetoma may form or an ocular infection may develop following penetrating trauma [1]. In humans, Acremonium spp. can lead to a wide range of infections from keratitis to disseminated disease, with the lung and gastrointestinal tract being the obvious portals of entry [3].
Acremonium spp infections in wild and domestic animals have also been described and include cutaneous lesions in Indian buffalo [4]. And in caimans [5]. keratoconjunctivitis [6]. And systemic infections [7,8]. In dogs; keratomycosis in cats [9]. And abortion in cattle [10]. Acremonium spp infection can be severe, with systemic damage to internal organs, including myocardium, pericardium, liver and kidneys, lymph nodes, endometrium and brain, and other internal organs. This has been shown in a description of a clinical case in a 4-year-old female German Shepherd [8]. Or a more recent study in a 2-year-old female Magyar Lop-Eared [7].
One of the first references of Acremonium spp skin infection in a dog was published in 1987, when dermatitis caused by it, accompanied by severe itching, alopecia, and subcutaneous nodules in various parts of the body, was described [11]. In the case of Acremonium spp infection in a French bulldog, the skin lesions and alopecia were not accompanied by signs of generalized infection, including enlarged lymph nodes and subcutaneous nodules.
The choice of itraconazole as a first-line antifungal agent was based on its affordability to the owner and its higher fungicidal activity compared to ketoconazole, fluconazole, and miconazole [1]. It should be noted that itraconazole may not be sufficiently effective against some strains of the pathogen. In one published study, itraconazole showed no activity and even voriconazole had a marginally effective minimum inhibitory concentration (MIC) against A. Kiliense [12]. Of the fungicides, voriconazole has the greatest in vitro activity against Acremonium and is recommended by European guidelines as an initial antifungal therapy [13]. Voriconazole has the best in vitro activity compared with amphotericin B, fluconazole and itraconazole against A. alabamensis and A. strictum [14]. Voriconazole and ravuconazole have similar activity and are superior to posaconazole against amphotericin B and itraconazole-resistant strains [15]. In this regard, voriconazole would be a better choice if available and without financial constraints.
We have included probiotics in the therapy regimen for the dog on the basis that they can enhance various defense mechanisms, modulate innate and adaptive immunity, neutralize toxins, carcinogens and pathogens, and release antioxidants and bacteriocins [16,17]. Suggested mechanisms for the health-promoting potential of probiotics include inhibition of pathogens and restoration of microbial homeostasis through microbial-microbial interactions, enhancement of epithelial barrier function, and modulation of the immune response [16,18,19].
The inclusion of probiotics in therapeutic regimens for mycoses is promising, as has been shown in several studies on candidiasis [16,20]. We used probiotic preparations containing B. Subtilis and Lactobacillus acidophilus, based on clinical findings of good tolerability and minimal risk of side effects. The fungistatic effects of B. subtilis and Lactobacillus acidophilus on Candida spp are known from the literature, including in skin and mucosal lesions [20,21]. Unfortunately, we were unable to find publications on the use of probiotics in the treatment of cutaneous Acremonium spp infection, and in this regard, the prescription of prosthetics was empirical. However, given the successful experience with probiotics in the treatment of other superficial mycoses, we consider this prescription to be justified. Although it should be noted that we cannot separately identify and assess the effect of prescribing probiotics in this clinical case.
To conclude the review of this clinical case, the combined sequential therapy with two fungicides, itraconazole and terbinafine, combined with topical antifungal and probiotic therapy for 8 weeks effectively controlled the infection. It should be remembered that, as with other fungal pathogens, the state of the host defense system, in particular the functional activity of neutrophils, is crucial for recovery [1, 22].
- Guarro J, Gams W, Pujol I, Gené J (1997) Acremonium species: New Emerging Fungal Opportunists-In vitro Antifungal Susceptibilities and Review. Clin Infect Dis 25: 1222-1229. Link: https://bit.ly/3rIoL7q
- Steinbach W.J. Miscellaneous Mycoses. In: Cherry J. et al. Feigin and Cherry's Textbook of Pediatric Infectious Diseases E-Book: 2-Volume Set. – Elsevier Health Sciences, 2013, 2111.
- Walsh TJ, Groll AH (1999) Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl Infect Dis 1: 247-261. Link: https://bit.ly/3EGM7zU
- Chatterjee A, Mitra K, Saha GR (1987) Isolation of Acremonium blochii (Matr.) W. Gams from cutaneous lesions in Asian buffaloes. Indian J Anim Health 26: 171-172.
- Trevino GS (1972) Cephalosporiosis in three caimans. J Wildl Dis 8: 384-388. Link: https://bit.ly/3v5AUWg
- Mendoza L, Donato A, Padhye A (1985) Canine mycotic keratoconjunctivitis caused by Acremonium kiliense. Sabouraudia 23: 447-450. Link: https://bit.ly/3v4pGkM
- Ballhausen BD, Geisweid K, Hartmann K, Hirschberger J, Majzoub M, et al. (2016) Systemic Acremonium species infection in a dog. Tierarztl Prax Ausg K Kleintiere Heimtiere 44: 424-428. Link: https://bit.ly/3EFRhw4
- Simpson KW, Khan KN, Podell M, Johnson SE, Wilkie DA (1993) Systemic mycosis caused by Acremonium sp in a dog. J Am Vet Med Assoc 203: 1296-1299. Link: https://bit.ly/3k01REr
- Binder DR, Sugrue JE, Herring IP (2011) Acremonium keratomycosis in a cat. Veterinary ophthalmology 14: 111-116. Link: https://bit.ly/3MppP8o
- Dion WM, Dukes TW (1979) Bovine mycotic abortion caused by Acremonium kiliense Grutz. Sabouraudia 17: 355-361. Link: https://bit.ly/3OolofT
- Gonzalez Cabo JF, Cequiel L, Solans Aisa C, Martinez Cortina A (1987) Isolation of Acremonium sp. in a case of canine dermatitis. Medicina Veterinaria (Spain) 4: 93-96. Link: https://bit.ly/3xK3mPa
- Radford SA, Johnson EM, Warnock DW (1997) In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrob Agents Chemother 41: 841-843. Link: https://bit.ly/3KaUhS2
- Tortorano AM, Richardson M, Roilides E, van Diepeningen A, Caira M, et al. (2014) ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect 20: 27-46. Link: https://bit.ly/3vAqKMf
- McGinnis MR, Pasarell L, Sutton DA, Fothergill AW, Cooper Jr CR, et al. (1998) In vitro activity of voriconazole against selected fungi. Med Mycol 36: 239-242. Link: https://bit.ly/3K5Qtl4
- Pfaller MA, Messer SA, Hollis RJ, Jones RN (2002) Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: Report from SENTRY antimicrobial surveillance program, 2000. Antimicrob Agents Chemother 46: 1032-1037. Link: https://bit.ly/3vf4rwZ
- Mailänder-Sánchez D, Wagener J, Schaller M (2012) Potential role of probiotic bacteria in the treatment and prevention of localised candidosis. Mycoses 55: 17-26. Link: https://bit.ly/3EGC6Cz
- Servin AL (2004) Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28: 405-440. Link: https://bit.ly/3OrlBii
- Boirivant M, Strober W (2007) The mechanism of action of probiotics. Curr Opin Gastroenterol 23: 679-692. Link: https://bit.ly/3K0QMgR
- Lebeer S, Vanderleyden J, De Keersmaecker SC (2008) Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev 72: 728-764. Link: https://bit.ly/3La4KOT
- Andrade JC, Kumar S, Kumar A, Černáková L, Rodrigues CF (2021) Application of probiotics in candidiasis management. Crit Rev Food Sci Nutr 1-16. Link: https://bit.ly/37DnuYv
- Zhao C, Lv X, Fu J, He C, Hua H, et al. (2016) In vitro inhibitory activity of probiotic products against oral candida species. J Appl Microbiol 121: 254-262. Link: https://bit.ly/3OwYinp
- Mueller RS (2007) Treatment of fungal infections. In: Dermatology for the Small Animal Practitioner- Teton New Media, Jackson, WY, USA. Link: https://bit.ly/3Mk1YH6
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.




 Save to Mendeley
Save to Mendeley
