International Journal of Veterinary Science and Research
Effects of phytogenic products on gut morpho-histology of broiler chickens
Megaache Mounia1*, Alloui Nadir2, Bennoune Omar1
2Poultry Science Division, Veterinary and Agricultural Sciences Institute, Batna University, LRESPA, Algeria
Cite this as
Mounia M, Nadir A, Omar B (2018) Effects of phytogenic products on gut morpho-histology of broiler chickens. Int J Vet Sci Res 4(1): 009-011. DOI: 10.17352/ijvsr.000028The objective of this experimental survey is to distinguish the effect of phytogenic products (Volarom®) as an alternative to antibiotic growth promoters (AGP) on the gut morpho-histology of broilers chickens, reared under optimal environmental conditions. Undred 1-day- Cobb strain chick males were divided into 2 groups (control and experimental). The broiler chickens received the following feed: control group: basic feed, experimental group: basic feed + 50 ml of phytogenic complex / 50 chicks in water for 42 days. At the age of 42 days, five birds per treatment were randomly selected and slaughtered. Representative segments of the gut broiler chickens (duodenum, jejunum and ileum) were removed from each slaughtered bird and then subjected to fixation and histological observation. Ten consecutive villi were analyzed, measuring their height and depth. Height was measured from the base of the villi at the junction of the crypt to the tip of the villi. For morphometry the software “Image J1-45s” was used Version 1.6.0_20. The gut morpho-histological structure of broiler chickens results indicated that the addition of 50 ml of phytogenic products / 50 chicks in water for 42 days decreased respectively the villi height of the duodenum, jejunum and ileum (1230 µm ± 270, 1127 µm ±290, 920 µm ± 220). However for the depth of crypts, the phytogenic effect is found only at the level of the duodenum (238 µm ± 50 vs 245 µm ± 60).
Statistically, there was no effect of the phytogenic products on the different parts of the broilers gut, in the experimental group at 42 days.
Introduction
Feed additives are products used in animal nutrition to improve the quality of food animal origin, or to increase the performance of production and health of animals [1]. However, the exaggerated use AGP has resulted in increased antibiotic residues in animal products and the development of antimicrobial resistance. This led, the European Union to ban the use of antibiotics growth promoters (AGP) in livestock production, in 2006. This has resulted in the search for alternatives to AGP in animal feeds, which will provide the same protection against pathogenic and non-pathogenic microorganisms, as well as improving the performance of animals. An intensive search for substitutes such as probiotics, prebiotics, symbiotic, enzymes, organic acids, organic minerals, oligosaccharides and other feed additives has taken place over the last decade [2]. Among the potential candidates, phytogenic represents a new and exciting group of feed additives, mainly from herbs, spices or other plants. Plant extracts and essential oils have received a great deal of attention because of their natural antimicrobial properties [3], to improve immune property [4], the ability to manipulate microorganism intestinals [1]; Also they have coccidiostatic activity [5,6]. This study tested the effect of the phytogenic complex (Volarom®) on the gut morpho-histology of broilers chickens at 42 days.
Material and Methods
Description of the experimental protocol
This study was conducted at the Experimental Poultry Station, Veterinary Department, Batna1 University, during September and October 2016. Animals are raised under optimized environmental conditions (temperature, humidity, air speed, ventilation). Experimental procedures followed the principles of the Animal Care Committee of the CRBt (National Center for Biotechnology Research), Constantine, Algeria. A total of 100 broiler chickens “Cobb”, male, aged 1day, were randomly allocated to 2 dietary treatments: one based on no feed additives (control), and one supplemented with 50 ml phytogenic products (Volarom®)/50 subjects in water (experimental). Feed formulation was carried out according to NRC (1996) [7], recommendations (Table 1). This phytogenic product is composed of a mixture of thyme, olive leaf, rosemary, grapefruit pepins and turmeric. Five birds per treatment were randomly selected and slaughtered, representative segments of the small intestine (duodenum, jejunum, and ileum) were taken from each bird and then subjected to fixation and histological segmentation as described by the method of [8].Ten consecutive villi were analyzed, measuring their height and depth [9]. For morphometry the software “Image J 1,45s” was used Version 1.6.0_20. The images are taken thanks to the use of a camera integrated into the microscope (Zeiss- Axioskope 20). The sensitivity of this camera is set to that of the film used (100 ASA).
Statistical analysis
All data were analyzed with Stat Soft software (Statistica 7, 2005). The statistically significant differences in treatment means were evaluated using one-way ANOVA, and a multiple comparison (Duncan) test was conducted with significant treatment means. P < 0.05 was considered as the significant level. Values are expressed as the means ± standard deviation (SD).
Results and Discussion
The results of gut morpho-histology for broilers slaughtered at the age of 42 days are shown in table 2, indicating villous height and crypt depth. Significant differences between dietary treatments (control and experimental) were measured for duodenal villus height and crypt depth (Figure 1). Villous height was longer for broiler chickens in the control group and shorter for broilers supplemented with the phytogenic products (PP). The depths of the duodenal crypt were significantly deeper in animals supplemented with PP, and shallower for broilers in the control group.
The size of the jejunum villi was longer in broiler chickens in the control group, while the broiler chickens receiving PP had the shortest jejunum villi. The depths of the jejunum crypt were shorter in broiler chickens supplemented with phytogenic products (PP) and deeper in broiler chickens in the control group (Figure 2). Statistically, there was no significant difference between villous size and crypt depth between control and experimental groups (p <0.05).
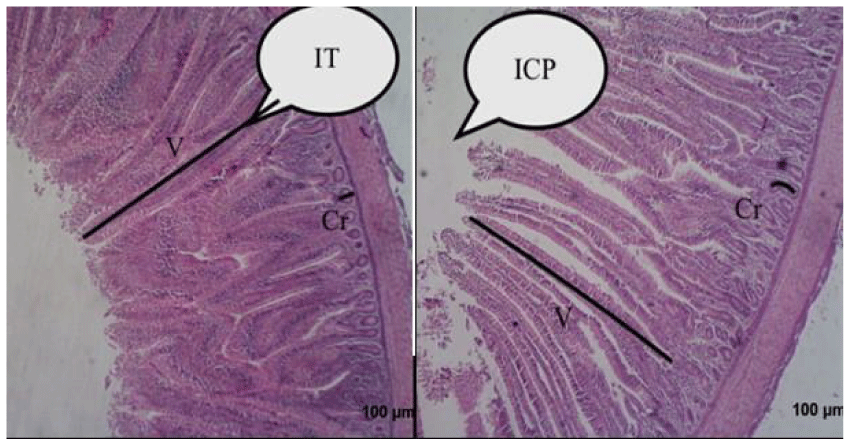
The results of the morphohistology of the ileum show that the broilers in the control group had the highest height and depth of the crypt compared to the experimental group (Figure 3). However, the difference between the 2 groups is only significant for the depth of crypts values (p <0.05).
Similar results have been found in studies by [10,11]. Recent study, revealed a significant increase in villous height, depth of crypt in broiler chickens supplemented with garlic powder at 0.5 g / kg or the combination garlic and black seed with a rate of 0.5 g / kg combined [12]. Improved intestinal morphology broilers, having consumed a ration supplemented with garlic powder or a combination of garlic powder and black seed also experienced a significant improvement in production performances [12].
Longer villi with more enterocyte cells for uptake and deeper crypts showing greater demand for new tissues due to proliferation in light have been found by [13,14]. A higher turnover of cells, thus a deeper crypt, would lead to a higher energy demand for maintenance of the digestive tract of the broiler. The energy could instead be used for growth and improved performance of animals [15]. Pathogenic bacteria present in the gastrointestinal tract are considered to damage enterocyte cells, which reduces their absorption, as well as deeper crypts [13]. Phytogenic feed additives are thought to reduce the number of pathogenic bacteria in the gastrointestinal tract [1,16], and would result in less damage to enterocytes and better uptake and villous height. However, many studies show no effect of phytogenic products on gut morphology and histology, making it difficult to link improved intestinal morphology to better animal performances.
Conclusion
The phytogenic product used in this experiment has an effect on the morpho-histological structure of the chicken gut (crypts and villi). However, the comparison of the results obtained between the experimental and the control group is not always statistically significant.
- Hashemi SR, Davoodi H (2011) Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet Res commun 35: 169-180. Link: https://goo.gl/Q8tVEa
- Fulton TM, Van der Hoeven R, Ennatta NT, Tankesley SD (2002) Identification, analysis, and utilization of conserved ortholog set markers for comparative genomics in higher plants. Plant Cell 14: 1457-1467. Link: https://goo.gl/voD5z6
- Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12: 564-582. Link: https://goo.gl/uTdmBk
- Guo FC, Savelkoul HFJ, Kwakkel RP, Williams BA, Verstegen MWA (2003) Immunoactive, medicinal properties of mushroom and herb polysaccharides and their potential use in chicken diets. World Poult Sci J 59: 427-440. Link: https://goo.gl/zAiqmv
- Allen P, Lydon J, Danforth H (1997) Effects of components of Artemisia annua on coccidia infections in chickens. Poult Sci Oxford University Press 76: 1156-1163. Link: https://goo.gl/BX5FA7
- Youn HJ, Noh JW (2001) Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Vet Parasitol 96: 257-263. Link: https://goo.gl/PCw2G9
- NRC (1996) Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources. Commission on Life Sciences, National Research Council, National Academy Press, Washington, D.C., USA. Link: https://goo.gl/g8EQ8V
- Iji PA, Saki A, Tivey DR (2001) Body and intestinal growth of broiler chiks on commercial starter diet development and characteristics of intestinal enzymes. Br poult sci 42: 514-522. Link: https://goo.gl/isZXi1
- Aptekmann KP, Baraaldi Arton SM, Stefanini MA, Orsi MA (2001) Morphometric analysis of the intestine of domestic quails (Coturnix coturnix japonica) treated with different levels of dietary calcium. Anat histol embryol 30: 277-280. Link: https://goo.gl/rHn4HR
- Peric? L, Miloševic? N, Žikic? D, Bjedov, S, Cvetkovic? D, Markov S, Mohnl M , Steiner, T (2010) Effects of probiotic and phytogenic products on performance, gut morphology and caecal microflora of broiler chickens. Archiv Tierzucht 53: 350-359. Link: https://goo.gl/UaQy5e
- Samadian F, Zeinoaldini S, Towhidi A, Amir M, Torshizi K, Pirasraei ZA ,Gholamzadeh P (2013) Evaluation of some phytogenic feed additives in growing chicks' diet. Intern J Agri Res and Review 3: 35-43. Link: https://goo.gl/vJ821T
- Saeid J M, Mohamed A B et Al-baddy M A (2013) Effect of adding Garlic Powder ( Allium sativum ) and Black Seed ( Nigella sativa ) in Feed on Broiler Growth Performance and Intestinal Wall Structure. J Nat Sci Res 3: 35-42. Link: https://goo.gl/vJ821T
- Parsaie S, Shariatmadari F, Zamiri M ,Khajeh K (2007) Influence of wheat-based diets supplemented with xylanase, bile acid and antibiotics on performance, digestive tract measurements and gut morphology of broilers compared with a maize-based diet. Br Poult Sci 48: 594-600. Link: https://goo.gl/CpVozu
- Xu Z, Hu C, Xia M, Zhan et Wang M (2003) Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. J Poult Sci 82: 1030-1036. Link: https://goo.gl/BHJwN3
- Choct M (2009) Managing gut health through nutrition. Br Poult Sci 50: 37-41. Link: https://goo.gl/JJCjsT
- Wenk C (2000) Herbs, spices and botanicals: Old fashioned' or the new feed additives for tomorrow's feed formulations? Concepts for their successful use. Biotech Feed Industry 200 79 -96. Link: https://goo.gl/JJCjsT
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Gut
Gut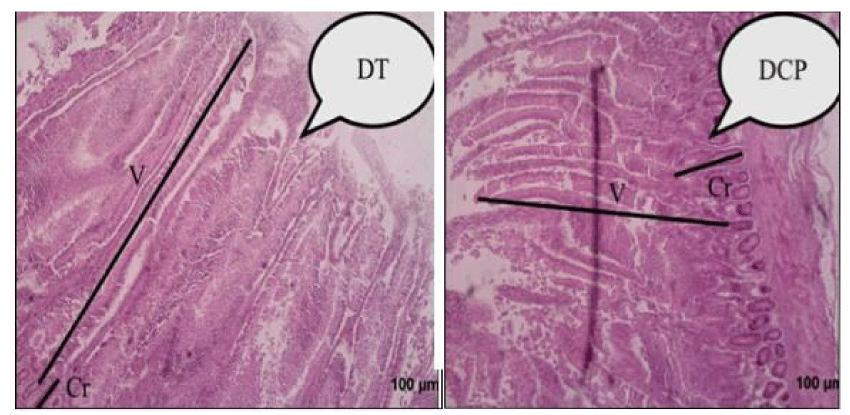


 Save to Mendeley
Save to Mendeley
