International Journal of Veterinary Science and Research
Study on Effect of Diatomaceous Earth (DAE) on Aflatoxin-Induced DNA Damage in Visceral and Lymphoid Organs in Broiler Chicken
AW Lakkawar1*, MI Satyanarayana2 and HD Narayanaswamy3
2Professor, Department of Pathology, Veterinary College, KVAFSU, Bangalore- 560024, Karnataka, India
3Professor and Associate Director of Research, Department of Pathology, Veterinary College, KVAFSU, Bangalore- 560024, Karnataka, India
Cite this as
Lakkawar AW, Satyanarayana MI, Narayanaswamy HD (2017) Study on Effect of Diatomaceous Earth (DAE) on Aflatoxin-Induced DNA Damage in Visceral and Lymphoid Organs in Broiler Chicken. Int J Vet Sci Res 3(2): 062-068. DOI: 10.17352/ijvsr.000023Background: Limited information exists concerning on the effect of diatomaceous earth (DAE) on aflatoxin-induced DNA damage in visceral and lymphoid organs.
Objectives: The present investigation is an attempt to detect the effect ability of Diatomaceous earth (DAE) in reducing the detrimental effects of aflatoxin (AF) in broiler diet was evaluated based on structural characteristic of DNA in liver, kidneys, heart, pancreas, thymus, spleen and bursa of Fabricius.
Materials and Methods: Three hundred and sixty healthy unsexed one day old broiler chicks were assigned to 9 groups comprising of control and treatment groups. DAE was supplemented @ 400 and 800 mg Kg-1 of feed along with 0.5 and 1 ppm of AF Kg-1 of feed for a period of 35 days. DNA fragmentation assay was conducted to detect changes in DNA.
Results: The DNA fragmentation in the toxin fed birds was severe in liver and lymphoid organs (thymus, spleen and bursa of Fabricius), followed by kidneys, heart and pancreas. The damage was more pronounced at 1 ppm in comparison to 0.5 ppm of dietary aflatoxin. The damage to DNA in most of the organs was reduced in birds of co-treatment groups fed with varying dosage of aflatoxin and DAE in the diet.
Conclusions: The present study showed that aflatoxin at graded doses induced marked DNA fragmentation indicating the genotoxic effect of aflatoxins. Addition of diatomaceous earth to aflatoxin mixed feed caused decreased DNA fragmentation in visceral and lymphoid organs.
Introduction
Aflatoxins (AF) are secondary metabolites and a class of mycotoxins produced predominantly by Aspergillus flavus and A. parasiticus [1]. The toxin occurs worldwide in feeds and feed stuffs resulting into severe economic loss to poultry and livestock industries in many countries around the world [2]. Studies have been related to negative effects of aflatoxin in broiler chickens including decrease in b ody weight gain, efficiency of feed utilization, liver damage, poor performance and immune responses. AF also caused pathologic alteration in important organs such as liver, kidneys and lymphoid organs [3]. The pathological change in broilers are characterized by hepatic lesions such as enlargement, paleness, fatty change, bile duct hyperplasia and periportal fibrosis [4]. Furthermore, the transmission of AF and its metabolites from feed to animal edible tissues and products, such as liver and eggs [5], becomes particularly important as a potential hazard for human health.
Producers and researchers desire to develop an effective detoxification technology dealing with the feed-borne toxin [6]. Approaches used included physical, chemical and biological treatment of contaminated feed and feed stuffs. A successful detoxification process must be economical and must be capable of eliminating all traces of toxin without leaving harmful residues and also should not impair the nutritional quality of the commodities [7]. In the last two decades several studies have been performed using adsorbent materials for detoxifying AF in contaminated feed and feed stuffs [8].
Several approaches to avoid contamination such as decontamination or remediation of feed and feedstuffs have been proposed [4]. A variety of adsorbents such as bentonite [9], zeolite [10], hydrated sodium calcium aluminosilicate [11], Saccharomyces cerevisiae [12], and activated charcoal [13], have been successfully used in detoxifying aflatoxins in contaminated feeds [14,15]. Adsorbent compounds utilized to ameliorate aflatoxicosis in poultry diets includes aluminosilicates, bentonite, silicas, zeolite, quartz, etc have been evaluated for their ability to remove or diminish the adverse effect of mycotoxins in animal feed. These compounds must not be absorbed from the gastrointestinal tract and must have the ability to bind physically with chemical substances, precluding their adsorption [10]. The major advantages of adsorbents include cost effectiveness, safety and easy administration through animal feeds. However, no compound is completely effective in eliminating aflatoxin from feed.
Genomic DNA constitutes the total genetic information of an organism. The genomes of almost all organisms are DNA, the only exceptions being some viruses that have RNA genomes. Genomic DNA molecules are generally large and in most organisms are organized into DNA-protein complexes called chromosomes. The size, number of chromosomes and nature of genomic DNA varies between different organisms. Aflatoxins possess genotoxic potential which is mainly due to adduct formation with DNA, RNA and protein. This may be the most important product from the carcinogenic point of view.
Diatomite or Diatomaceous earth (DAE) is a kind of clay that consists of 90 per cent silicon dioxide. It is fine-grained, biogenic siliceous sediment and is available in large quantities at low cost [16]. DAE consists essentially of amorphous silica derived from opalescent frustules of diatoms resulting in an inert, light weight, highly porous, super-absorbent material, and has a fine porous structure with low density [17]. Modirsanei et al. [18], reported that diatomaceous earth increased the body weight gain, feed intake, and improved the feed conversion ratio as well as productive efficiency index. DAE also increased serum albumin levels in the birds that were subjected to AF supplementation.
Considering the beneficial properties of diatomaceous earth, the present study was undertaken to evaluate the efficacy of DAE in ameliorating aflatoxin induced patho-morphological changes in broilers.
Materials and Methods
Aflatoxins
Aflatoxin (AF) was produced on the rice using Aspergillus parasiticus (MTCC-2796) as per the method of Shotwell et al. [19], with some modifications and was quantified using thin layer chromatography.
Experimental birds and diet
Three hundred and sixty unsexed day-old healthy broiler chicks were procured from a reputed commercial hatchery and reared in battery cage system in experimental sheds with average temperature ranging from 27 to 31oC and relative humidity of 59% to 62% with 16:8±I h Light : Dark cycle of intensity of 10 to 20 lux. All chicks were vaccinated on days 7 and 11 of age with the Lasota strain of Newcastle disease virus and Infectious bursal disease (intermediate strain) respectively.
Optimum conditions of brooding and management were provided to the birds throughout the period of experiment. Toxin free and Diatomaceous earth (DAE) free Starter and finisher broiler feed was procured from Department of Poultry Science, Veterinary College, Hebbal, Bangalore-24, India as recommended by the National Research Council. Required quantity of cultured aflatoxin material was added to make the final concentration of aflatoxin in feed to be 0.5 ppm and 1ppm.
The birds were randomly divided into 9 groups, each comprising of 40 chicks. The different experimental group are as per the table 1.
All the birds were checked daily for the health and husbandry conditions. All the sanitary and hygienic precautions were strictly followed throughout the experiment. Prior permission of the Institute Animal Ethical Committee (IAEC) was obtained before the conduct of experiment. The birds were observed daily for clinical signs of aflatoxicosis characterised by dullness, poor growth, inappetence, diarrhoea and mortality (if any). A complete record of the daily mortality (if any) was also maintained.
Sample collection
At the end of the experimental period, the birds were euthanized by cervical dislocation and were subjected to detailed post-mortem examination. Representative tissue samples from liver, kidneys, pancreas, intestines, heart, thymus, bursa of Fabricius were collected, put on ice and immediately carried to the laboratory for DNA extraction.
Extraction of tissue DNA
The tissue DNA from liver, kidneys, heart, pancreas, thymus, spleen and bursa of Fabricius of birds belonging to all the groups was extracted as per the standard protocol, using the DNeasy® Blood and Tissue kit procured from Qiagen, Inc., (USA).
Protocol for DNA extraction
a) The tissues (≤ 10 mg spleen or ≤ 25 mg other tissue) were cut into small pieces, and placed in 1.5 ml microcentrifuge tube.180 μl Animal Tissue lysis (ATL) buffer and 20 μl proteinase K were added and mixed by vortexing for 4-5 seconds, and incubated at 56oC until completely lysed. Vortexing was done occasionally during incubation.
b) 200 μl of Lysis buffer (AL) was added and mixed thoroughly by vortexing by pulse-vortexing for 5–10 seconds.
c) Two hundred microlitre of absolute ethanol (99%) was added to the sample and mixed by pulse-vortexing for 15 seconds.
d) After mixing, the microcentrifuge tube was briefly centrifuged at 3000 rpm for 1 minute to remove the drops from inside the lid.
e) The mixture from the above step (including the precipitate) was carefully applied to the QIAamp mini spin column along with 2 ml collection tube without wetting the rim. The cap was closed and centrifuged at ≥ 6000 x g for 1 min. Spin column was placed in a new clean 2 ml collection tube and collection tube containing the filtrate was discarded.
f) 500 microlitre of wash buffer (AW1) was carefully added without wetting the rim of the spin column.
g) The cap was closed and centrifuged at ≥ 6000 x g for one minute and the collection tube was discarded.
h) The spin columns were carefully opened and 500 μl of wash buffer (AW2) was added without wetting the rim of the spin column.
i) The cap was closed and centrifuged at 20,000 x g for 3 minute.
j) QIAamp mini spin column was placed in a clean 1.5 ml microcentrifuge tube and 200 μl elution buffer (AE) or distilled water was carefully added and incubated at room temperature for one minute. Finally, the DNA was eluted at ≥ 6000 x g for 2 minute.
Determination of purity and yield of the DNA samples
The purity and concentration of the extracted tissue DNA was estimated by UV spectrophotometry. An aliquot of 20 µl of DNA sample was dissolved in 0.98 ml of sterile distilled water. The diluted DNA was transferred into 1ml microcuvette and the optical density (OD) was checked at 260 nm and 280 nm in a UV spectrophotometer. Sterile distilled water was used as blank. The ratio of OD at 260/280 nm was calculated. A ratio of 1.7 to 1.9 was considered pure. The concentration of the DNA was estimated by the equation: 1 OD 260 nm = 50 μg/ml of DNA. Further, the purity of the DNA sample was checked by electrophoresis on 1.8 per cent agarose gel.
Protocol for DNA confirmation and fragmentation study By Agarose Gel Electrophoresis
For electrophoresis, 1.2 % agarose was used and it was carried out as follows.
a) The edges of a clean, dry, gel casting tray sealed at both the ends using adhesive tape. An appropriate comb was placed to form a sample slot in the gel.
b) Agarose solution was prepared by dissolving required quantity of agarose in proportionate volume of 1X Tris-Borate-Ethylenediaminetetraacetic acid (TBE) buffer and melted in a microwave oven for 1 min.
c) Once the molten gel cooled, 0.5 μg of ethidium bromide was added and mixed thoroughly by gentle swirling.
d) Warm agarose solution was then poured into the gel casting tray avoiding formation of air bubbles and allowed to solidify.
e) Once agar gel solidified, a small amount of Tris-acetate- Ethylenediaminetetraacetic acid (TAE) buffer was poured on the top of the gel to remove the comb. Then the buffer was poured off and the tape was removed.
f) The gel casting tray was mounted in the electrophoresis tank and the TAE buffer was added just enough to cover the gel to a depth of one mm.
g) 5 μl of DNA was mixed with 1/6th volume of 6X gel loading dye and slowly loaded into the slots of submerged gel using a micropipette.
h) The gel tank was closed with the lid and electrical leads were attached so that the DNA would migrate towards the anode.
i) The electrophoresis was carried out at 5V / cm at room temperature (RT) until the bromophenol blue dye migrated appropriate distance through the gel.
j) Following electrophoresis, DNA bands were visualized at 300 nm wavelengths using a UV transilluminator and the images were captured by using Gel Doc XR (Bio Rad, USA) for further interpretation.
Result
The results of quantification of isolated DNA from various organs are expressed in ng/µl by Nanodrop method using spectrophotometer has been summarized in tables 2,3. The fragmentation of DNA in various tissues of broiler chickens in different treatment groups at the end of experiment (35th day) has been analysed and the same is presented as below.
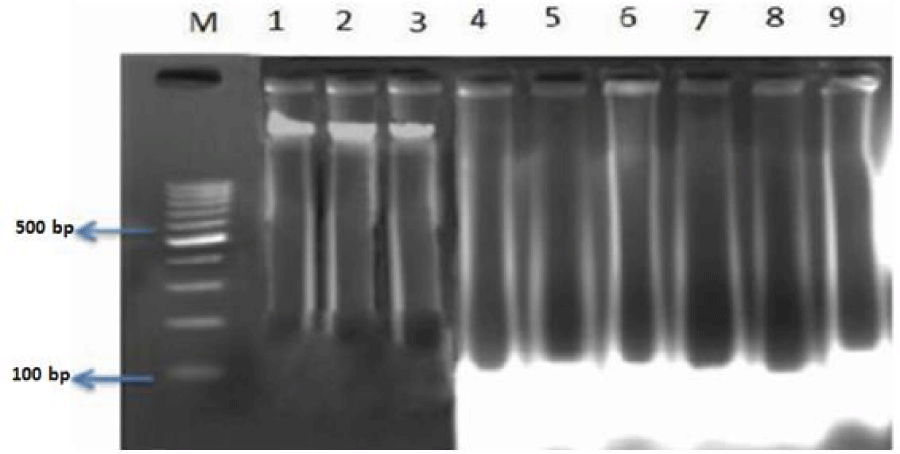
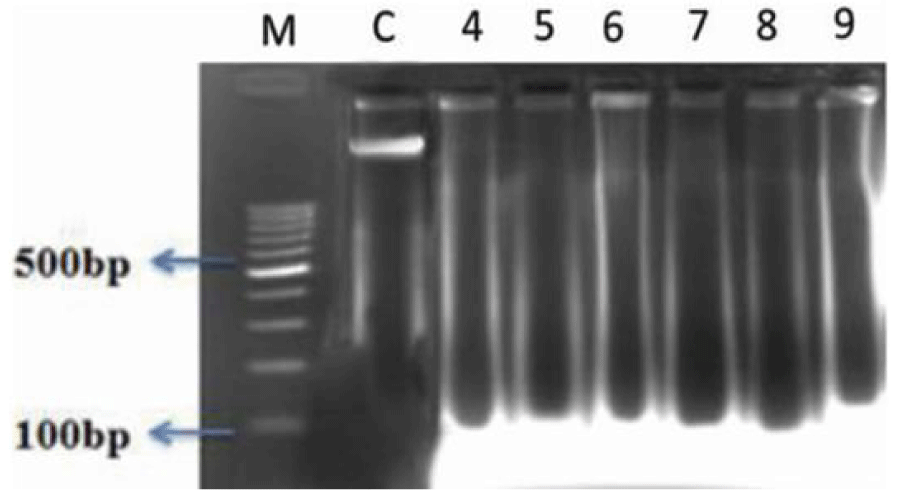
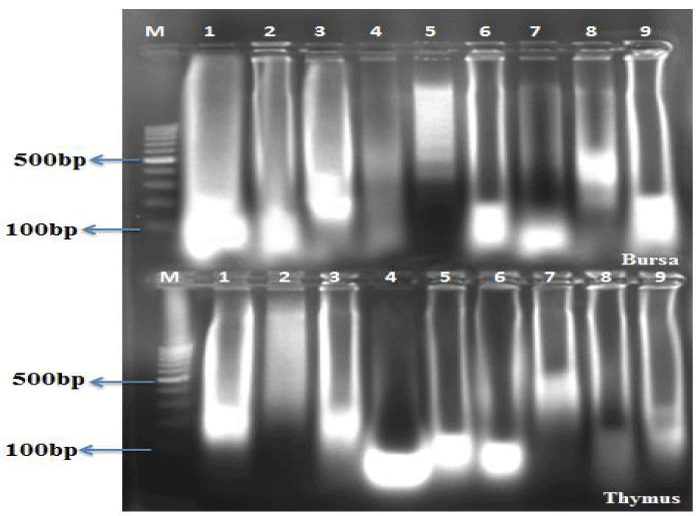
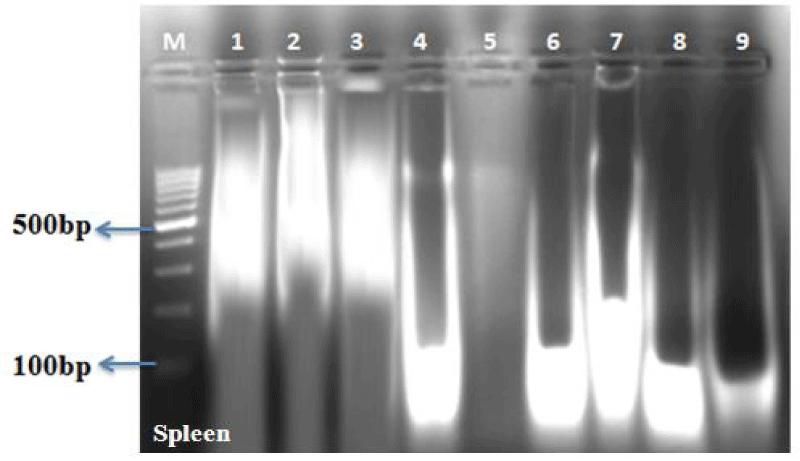
The DNA fragmentation in AF toxin fed groups was observed in all the organs examined such as liver (Figure 1, Table 4), kidneys (Figure 2, Table 5), thymus (Figure 3, Table 6), bursa (Figure 3, Table 6) and spleen (Figure 4, Table 7).
AF feeding caused severe damage to DNA of liver and lymphoid organs namely thymus, spleen and bursa of Fabricius; followed by kidneys and the least damage was observed to the DNA of heart and pancreas. The damage was more pronounced at 1 ppm (Group IV) in comparison to 0.5 ppm (Group V) of dietary aflatoxin.
The DNA from various tissues of birds fed diet free of aflatoxin (Group I) and DAE alone to toxin free feed (Group II and III) showed no damage to the DNA.
The damage to DNA in most of the visceral and lymphoid organs was significantly reduced in bird of co-treatment groups fed 0.5 and 1ppm of aflatoxin and DAE @ 400 and 800 mg/kg feed (Group VI to IX). The birds fed with DAE @ 800 mg/kg feed and aflatoxin (0.5 and 1 ppm) showed reduced damage to DNA in comparison to broilers fed with DAE @ 400 mg/kg feed and aflatoxin @ 0.5 and 1 ppm.
Discussion
Genomic DNA constitutes the total genetic information of an organism. The genomic DNA in cells can be subjected to injury following interaction due to exposure to toxicants. Mycotoxins, especially the aflatoxins are known to cause interaction with the cellular total genomic DNA [20,21]. This interaction can result in small to large changes in genomic DNA evident as DNA fragmentation [22], similar to the findings of the present study.
The fragmentation assay of DNA extracted from heart, liver, kidneys, pancreas, thymus, spleen and bursa of birds fed with different dietary levels of aflatoxin in the present study revealed higher damage at 1 ppm in comparison to 0.5 ppm. Following aflatoxin treatment, severe damage caused to DNA of liver and lymphoid organs, followed by kidneys and the least damage was observed in DNA of heart and pancreas in comparison to negative control groups. The liver is a target organ for AF toxicity as it is the site where aflatoxins undergo bioactivation to reactive 8,9-epoxide, which then binds to DNA and proteins [23], causing wide spread damage to the hepatic tissue. In addition, aflatoxins have direct tropism for any of the primary or secondary lymphoid organs resulting into either the organ necrosis or atrophy or depletion of specific subpopulations of the lymphoid cellular components [24].
Similar to the findings in the present study, Shebl et al. [25], reported the higher mean percentages of DNA fragmentation and increase in the frequency of micronucleated cells in liver cells following feeding of aflatoxin @ 211.88 µg / kg feed to the broiler in comparison to negative control groups and opined that AFB1 was selective inhibitor of DNA synthesis in mammalian cells and can induce DNA adducts, induce mutations by intercalating to DNA by forming adduct with guanine moiety in the DNA. Similar findings were also reported by earlier workers following mycotoxicosis [26,27].
Al-Terehi [28], observed higher degree of DNA fragmentation in liver and spleen in comparison to kidney and blood in female albino rats following feeding of aflatoxin for a period of 2 weeks. The DNA damage observed in various organs could be attributed to AFB1- DNA adduct formation at cellular level, as suggested by Tolliver and Robbins [29]. In addition, oxidative stress to rats following exposure to AFB1 can also induce DNA lyses by forming (8-oxo d G) in liver cells [30].
Aflatoxin possess genotoxic potential through the mixed-function oxidase system to a number of hydroxylated metabolites and to aflatoxin 8, 9 epoxide which binds to DNA, forming covalent adducts [31], and disturbs DNA replication causing genetic alteration [32]. The adduct formation occurs preferably with guanine resulting in AFB1-N-7 guanine adduct responsible for mutagenesis in AFB1 treated cells [33].
DNA fragmentation test in the present study clearly indicated that aflatoxin could not damage the nucleus of cells in different organs of DAE supplemented birds (Group VI to IX), which might be explained by the strong adsorption of AF to diatomaceous earth and thereby preventing the entry of AF into circulation [27]. Similar to the findings of present study, Shebl et al. (2010) [25], also reported that the addition of clay material (DAE) either alone or in combination with aflatoxin reduced the mean percentages of DNA fragmentation in different organs.
The effectiveness of compound in sequestering one mycotoxin does not mean an equal ability to sequester other mycotoxins due to chemical complexity of these toxins. Each of the mycotoxin has different functional groups; thus, the binding capacity of an adsorbent depends on its chemical and physical properties and its relation with the physical structure of the target mycotoxins. Thus, the physicochemical differences among the adsorbents used in the studies mentioned above could explain the higher or lower efficacy among them. However, the ability of the toxin binder to bind mycotoxins depends on other factors such as pH, molecular arrangement and its geographic region of origin [34]. Natour and Yousef [35], reported significantly higher in-vitro adsorption ability of DAE to aflatoxin, which is directly proportional to the number of diatom valves. Diatomaceous earth being a powerful natural adsorbent can effectively adsorb the mycotoxins through its polar ends [36]. In addition, DAE has a small mass (0.5-0.8 g/cm3), high porosity and high content of silicon responsible for the high adsorption capacity [37]. In- vitro study showed that DAE has high (94.71 %) ability to absorb AF from the feed at pH 6.5 [38]. The normal pH of the chicken intestinal tract contents is 5.7–6.0 in the duodenum/jejunum, 6.3–6.4 in the ileum/rectum and finally up to pH 7.0 or higher in the caecum (Denbow, 2000) [39]. Considering the correlation between the pH and ability of mycotoxin binder in in-vitro studies, higher binding ability of DAE to the aflatoxins can plausibly be expected at the pH of 6-7 in the intestinal tract of chicken to reduce the absorption and systemic availability of this mycotoxin.
In conclusion, the incorporation of DAE in the diet during the period of exposure to AF in the present study could considerably reduce the toxic effects of aflatoxin. This study highlights the protective effects of DAE, which might be due to its capability of specific chemisorption of aflatoxin in gastrointestinal tract, which reduces AF bioavailability by formation of aflatoxin-DAE complex followed by excretion through droppings/faeces [40,41].
The compounds like clay, zeolite minerals and DAE are structurally and functionally diverse; they vary considerably from source to source and may not have equal affinities and capacities for binding of aflatoxin and other mycotoxins. Thus these adsorbents should be rigorously tested one by one and thoroughly in in-vivo conditions, paying particular attention to their effectiveness and safety in sensitive animal/avian models and their potential for harmful interactions. Similarly, generalisations should be avoided for all potential mycotoxins detoxifying agents, as adsorbing compounds can differ in efficacy even within the same category. Considering the results of present study and earlier work done on effect of different levels of DAE on aflatoxin, further studies employing the broader perspectives seems to be necessary to determine whether lower levels of DAE in broilers diet will be effective in controlling or preventing the occurrence of aflatoxicosis in chicken.
Authors are the thankful to the Vice Chancellor, KVAFSU, Bidar, Dean, Veterinary College, Bangalore, ICAR-NAE Project for providing the necessary facilities. Thanks are due to Agripower, Australia for providing DAE for study purpose and financial support to carry out the research work.
- Huwig A, Freiund S, Kappeli O, Dutler H (2001) Mycotoxin detoxication of animal feed by different adsorbents. Toxicol Lett 122: 179-188. Link: https://goo.gl/KnaAWR
- Miller JD (1995) Fungi and mycotoxins in grain: Implications for stored product research. J Stored Prod Res 31: 1-16. Link: https://goo.gl/ScwGui
- Dafalla R, Yagi A, Adam SE (1987) Experimental aflatoxicosis in hybro-type chicks; sequential changes in growth and serum constituents and histopathological changes. Vet Human Toxicol 29: 222-226. Link: https://goo.gl/4udMA6
- Ledoux DR, Rottinghaus GE, Bormudez AJ, Alonsodebolt M (1999) Efficacy of sodium calcium alumino silicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poult Sci 78: 204-210. Link: https://goo.gl/2YDmtK
- Bintvihok A, Thiengnin S Doi K, Kumagai S (2002) Residues of aflatoxins in the liver, muscle and eggs of domestic fowls. J Vet Med Sci 64:1037–1039. Link: https://goo.gl/a2hRAX
- Leeson S, Diaz G, Summers JD (1995) Aflatoxins. In Leeson S, Diaz G, Summers JD (eds), Poultry Metabolic Disorders and Mycotoxins, University Books, Ontario, Canada 248–279. Link: https://goo.gl/yiXKsq
- Parlat SS, Yildiz AO, Oguz H (1999) Effect of clinoptilolite on performance of japanese quail (Conturnix conturnix japonica) during experimental aflatoxicosis. Br Poult Sci 40: 495-500. Link: https://goo.gl/Qiv9Vr
- Abo-Norag M, Edrington TS, Kubena LF, Harvey RB, Phillips TD (1995) Influence of hydrated sodium calcium aluminosilicate and virginiamycin on aflatoxicosis in broiler chicks. Poult Sci 74: 626-632. Link: https://goo.gl/xdM1Po
- Rosa CAR, Miazo R, Oli C, Salvano M, Chiacchier SM, et al. (2001) Evaluation of the dietary effect of bentonite from the south of Argentina in ameliorating the toxic effects of aflatoxin in broilers. Poult Sci 80: 139-144. Link: https://goo.gl/9osdZT
- Miazzo R, Rosa CA, De Queiroz Carvalho EC, Magnoli C, Chiacchiera SM, et al. (2000) Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poult Sci 79: 1–6. Link: https://goo.gl/WqaMLC
- Scheideler SE (1993) Effects of various types of aluminosilicates and aflatoxin B1 on aflatoxin toxicity, chick performance and mineral status. Poult Sci 372: 282-288. Link: https://goo.gl/MKAhn6
- Celik K, Denli M, Erturk M, Ozturkcan O, Doran F (2001) Evaluation of dry yeast Saccharomyces Cerevisiae in the feed to reduce aflatoxin B1 residues and toxicity to Japanese quails (Coturnix coturnix Japonica). J Appl Anim Res 20: 245-250. Link: https://goo.gl/whwyRD
- Jindal N, Mahipal SK, Mahajan NK (1994) Toxicity of aflatoxin B1 in broiler chickens and its reduction by activated charcoal. Res Vet Sci 56: 37-40. Link: https://goo.gl/dF9Ycp
- Ramos AJ, Hernandez E (1997) Prevention of aflatoxicosis in farm animals by means of hydrated sodium calcium aluminosilicate addition to feedstuff: A review. Anim Feed Sci Technol 65: 197-206. Link: https://goo.gl/rNphr7
- Jia-Sheng W, John DG (1999) DNA damage by mycotoxins. Mutation Res 424: 167-181. Link: https://goo.gl/5SgSk1
- Kamigasa S, Kato H (2000) Recent conditions and prospects of diatomite resources. Energy Resources 21: 166-172.
- Wajima T, Haga M, Kuzawa K, Ishimoto H, Tamada O, et al. (2006) Zeolite synthesis from paper sludge ash at low temperature (90 degrees C) with addition of diatomite. J Hazardous Materials; 132: 244-252. Link: https://goo.gl/oX43Vc
- Modirsanei M, Mansoori B, Khosravi AR, Kiaei MM, Khazraeinia P, et al. (2008) Effect of diatomaceous earth on the performance and blood variables of broiler chicks during experimental aflatoxicosis. J Sci Food Agric 88: 626-632. Link: https://goo.gl/bSiQDS
- Shotwell OL, Hesseltine CW, Stubblefeild RD, Sorenson WG (1966) Production of aflatoxin on rice. Appl Microbiol 52: 425-428. Link: https://goo.gl/YyVECX
- Williamson R (1970) Properties of Rapidly Labelled Deoxyribonucleic Acid Fragments Isolated from the Cytoplasm of Primary Cultures of Embryonic Mouse Liver Cells. J Mol Biol 51: 157-168. Link: https://goo.gl/aCWvsG
- Choy WN (1993) A review of the dose-response induction of DNA adducts by aflatoxin B1 and its implications to quantitative cancer-risk assessment. Mutation Res 296: 181-198. Link: https://goo.gl/EwUQVX
- Faridha A, Fasial K, Akbarsha MA (2006) Duration dependent histopathological and histometric changes in the testis of aflatoxin B1-treated mice. J Endocrinol Reprod 10: 117-133. Link: https://goo.gl/S3FDrF
- Qureshi MA, Hussain I, Heggen CL (1998) Understanding immunology in disease development and control. Poult Sci 77:1126-1129. Link: https://goo.gl/fMnfmY
- Pasha TN, Farooq MU, Khattak FM, Jabbar MA, Khan AD (2007) Effectiveness of sodium bentonite and two commercial products as aflatoxin absorbents in diets for broiler chickens. Anim Feed Sci Technol 132: 103– 110. Link: https://goo.gl/1MRf8U
- Shebl MA, Hafiz NA, Motawe HFA (2010) Genotoxic Studies of yeast Cell Wall (YCW) and Hydrated Sodium Calcium Aluminosilicate (HSCAS) on the DNA Damage and Chromosomal Aberrations Induced by Aflatoxin in Broiler. J American Sci 6: 961-967. Link: https://goo.gl/1zgCYU
- Smela ME, Curier SS, Bailey EA, Essingmann JM (2001) The chemistry and biology of aflatoxin B(1): from mutational spectrometry to carcinogenesis. Carcinogenesis 22: 535-545. Link: https://goo.gl/pQifvg
- Abbès S, Salah-abbès JB, Hetta MM, Ibrahim M, Abdel-Wahhab MA, et al. (2008) Efficacy of Tunisian montmorillonite for in-vitro aflatoxin binding and in-vivo amelioration of physiological alterations. Appl Clay Sci 42:151–157. Link: https://goo.gl/j9cik4
- AL-Terehi MN (2012) Effect of aflatoxin B1 on DNA structural characteristic. Res Pharmacy 2: 35-38. Link: https://goo.gl/JwPdL1
- Tolliver D, Robbins L (1991) Techniques in karyology: the bone marrow extraction method. Asso Biol Lab Edu 12: 69-73. Link: https://goo.gl/LuwT9Z
- Shen HM, Ong CN, Lee BL, Shi CY (1995) Aflatoxin B1 induced 8- hydroxyldeoxyguanosine formation in rat hepatic DNA. Carcinogenesis 16: 419-422. Link: https://goo.gl/z3pqRh
- Busby WF Jr, Wogan GN (1984) Aflatoxins. In: Searle, C. E. (ed.). Chemical carcinogens, 2nd ed. ACS Monogragh 182. American Chemical Society, Washington DC 945-1136.
- Preston RJ, Williams GM (2005) DNA-reactive carcinogen: mode of action and human cancer hazard. Crit Rev Toxicol 35: 673-683. Link: https://goo.gl/xAFMsw
- Kallio M, Lahdetie J (1997) Effects of the DNA topoisomerase II inhibitor merbarone in male mouse meiotic divisions in vivo: Cell cycle arrest and induction of aneuploidy. Environ Mol Mutagenesis 29: 16-27. Link: https://goo.gl/jegtEK
- Vieira SL (2003) Nutritional implication of mould development in feedstuff and alternatives to reduce the mycotoxins problem in poultry feed. World's Poult Sci J 59: 111-122. Link: https://goo.gl/iBRMff
- Natour RM, Yousef SM (1998) Adsorption efficiency of diatomaceous earth for mycotoxin. Arab Gulf Journal of Scientific Research 16: 113–127. Link: https://goo.gl/Wb2VH7
- Gowda NKS, Ledoux DR (2008) Use of Antioxidants in Amelioration of Mycotoxin Toxicity: A Review. Anim Feed SciTechnol 8: 1-11. Link: https://goo.gl/wCDcku
- Whitlow LW (2006) Evaluation of mycotoxin binders. 4th Mid-Atlantic Nutrition Conference (Zimmerman, N.G. ed.), Proceedings 132-143. Link: https://goo.gl/aVGGVL
- Soleimani R, Faradonbeh OP, Bagheri H (2011) Mycotoxin detoxification of commercial broiler feed by a mycotoxin binder. Res Opinions Anim Vet Sci 1: 778-780.
- Denbow D (2000) Gastrointestinal anatomy and physiology. In: Whittow GC, ed. Sturkie's Avian Physiology. 5th edn. Academic Press, San Diego, CA, USA 299-324.
- Phillips TD (1999) Dietary clay in the chemoprevention of aflatoxin induced disease. Toxicol Sci 52 (Suppl): 118 – 126. Link: https://goo.gl/rY1HRVAbdel-Wahhab MA, Nada SA, Amra HA (1999) Effect of aluminosilicates and bentonite on aflatoxin-induced developmental toxicity in rat. J Appl Toxicol 19: 199–204. Link: https://goo.gl/vaVj8r
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
