International Journal of Veterinary Science and Research
Freshwater Snail Distribution Related to Physicochemical Parameters and Aquatic Macrophytes in Giza and Kafr El-Shiekh Governorates, Egypt
Fatma AA El Deeb1, Nahla S El-Shenawy2*, Maha FM Soliman2 and Sara A Mansour1
2Zoology Department, Faculty of Science, Suez Canal University, Ismailia 41522, Egypt
Cite this as
El Deeb FAA, El-Shenawy NS, Soliman MFM, Mansour SA (2017) Freshwater Snail Distribution Related to Physicochemical Parameters and Aquatic Macrophytes in Giza and Kafr El-Shiekh Governorates, Egypt. Int J Vet Sci Res 3(1): 008-013. DOI: 10.17352/ijvsr.000015A field work was conducted to study the density of the freshwater snail in relation to the vegetation cover as well as the physical and chemical properties in different watercourses. Two sites were selected in Giza governorate while, three sites were selected at Kafr El-Shiekh governorate. Water temperature, conductivity, total hardness, and pH were measured in the selected sites as well as bisphenol A (BPA) levels. Snail sampling was carried out and all types of macrophytes found in each site were collected, identified and coverage. Nine snails species namely Biomphalaria alexandrina, Physa acuta, Planorbis planorbis, Lymnaea natalensis, Bulinus truncates, Bellamya unicolor, Melanoides tuberculata, Helisoma duryi and Lanistes carinatus were identified. B. alexandrina was the most enumeration of snail species. The percentage of total snail species (75.47 %) was recorded at 28 °C as compared to 5.86 % recorded at 34 °C. Five species of aquatic vegetation were identified, two of them correlated positively and significantly with various snail species. Sites in which snails associated with macrophytes were characterized with higher ranges of chemicals, dissolved oxygen, and conductivity. In conclusion, the most important of the associating vegetation was L. gibba which correlated with B. alexandrina and served as an indicator plant.
Introduction
The freshwater ecosystem is under increasing threat due to rapidly expanding population and the subsequent modernization process resulted in inconspicuous exploitation of nature leading to the pollution crisis. Rivers are vulnerable since waste effluents from industries, domestic and farms open directly into them. Industrial and domestic effluents which account for the pollution that endangers the aquatic life contain various toxic substances [1].
Bisphenol A [4,4′-(propane-2,2-diyl) diphenol; BPA] is one of the environmental contaminants widely used in the manufacture of polycarbonate plastic and epoxy resins [2]. Bisphenol A (BPA) is a pseudo-persistent chemical, which despite its short half-life is ubiquitous in the environment because of continuous release [3]. Surface-water concentrations of BPA vary considerably depending on the location, sampling period, and how the results are reported. It has noted that although BPA dissolved in surface water has a short half-life because of photo and microbial degradation, while, metabolites may persist [4]. The most values reported for BPA in surface water are below 1.0 mg/L [4]; BPA concentrations can vary with depth [5].
Submersed macrophytes like Ceratophyllum demersum and Ceratophyllum demersum have major effects on productivity and biogeochemical cycles in freshwater because they occupy key interfaces in stream and lake ecosystems. Moreover, some aquatic plants are known to accumulate industrial radionuclides and heavy metals [6]. Freshwater pulmonate snails are commonly found in association with macrophylic vegetation and their epiphyton [7]. These macrophytes provide sites for snail oviposition, access to the air-water interface and shelter, and provide a surface for epiphyton development, which constitutes a major source of the food of freshwater snail [8]. It has been proposed that the close association of plants and snails in freshwater habitats since the cretaceous may have led to the development of mutually beneficial interactions [8]. This hypothesis is supported by laboratory experiments which show that the presence of freshwater snails can increase macrophytes growth and leaf longevity [9]. This effect appears to be due to both nutrient exchange and removal of epiphyton by feeding snails [10].
The mechanisms of competition between Melanoides tuberculatus and Biomphalaria sp. are not yet understood, but the competition for food probably occurs because these snails have a similar diet, including fine detritus and epiphytic algae [11, 12]. M. tuberculatus is capable of reaching high densities; hence, competition for space is also possible [13]. However, the outcome of the interactions between M. tuberculatus and Biomphalaria spp. seems to be related to the habitat type where both species occur. In a former study in Kenya [14], no evidence was found of negative effects due to the presence of M. tuberculatus on Biomphalaria spp. Populations [15].
Continuous field studies are needed to detect changes in the distribution and abundance of the snails that are due to global climate and ecological changes [16]. The Egyptian freshwater habitat has been deteriorating primarily due to the discharge of municipal wastewater, industrial and agricultural into various water bodies across the country.
A field work was conducted to study the possible relation between the distribution of the freshwater snail with BPA concentrations. The correlation of snails with the density as well as the types of aquatic plants was determined in different watercourses in Giza and Kafr El-Sheikh governorates.
Materials and Methods
Study area: This study was conducted in two Egyptian governorates; Giza and Kafr El Shiekh. Some selected sites were chosen from different centers in each governorate. At Giza governorate, two sites were chosen namely El-Salmawy and Kafr Hakim (Figure 1). At Kafr El-Shiekh governorate, three sites were selected namely Qulin, Shinu and Kafr EL-Shiekh (Figure 2). The watercourses included irrigation canals and agricultural drains.
Physical and chemical parameters of water: Water temperature, conductivity, dissolved oxygen (DO) and total dissolved solid (TDS) were measured directly in the selected watercourses to the nearest oC, μs /m, ppm, and mg/L, respectively using temperature conductivity meter (HANNA instrument, HI 9635) and a portable D.O. meter (HI 8543). Moreover, hydrogen ion concentration (pH) was measured by pH meter electrode (HI 9124 and HI 9125). All the physical parameters were measured between 11: 00 am to 3: 00 pm and were recorded in the field survey sheets.
Snail survey: Snail sampling was carried out during spring 2013 and sampling was performed using a standard dip net (33 x 33 Cm) [17]. At each sampling site, three adjacent dip nets were taken, covering a length of about one meter. The collected snails were sorted and recorded in field survey sheet [18].
Aquatic plants: All types of macrophytes found in each site were collected, properly labeled and identified to species level. Coverage was determined with some modification by a simple estimation of the proportion of site covered by each species and scored 1 for ≤ + (very low coverage), 2 for ++ (low coverage), 3 for +++ (moderate coverage), 4 for ++++ (high coverage) and 5 for ≥ +++++ (very high coverage) according to [19].
Determination of BPA in water sample: The extraction and analysis of BPA from collected water sample were done using HPLC system equipped with Smart line pump 1000, UV detector model basic 2500 set as 230 nm and model YL9100 Berlin manual injector with a 20 μL sample loop. The system used for this work was equipped with two detectors in series, ultraviolet (230 nm) and fluorescence (Ex 225 nm, Em 310 nm). Sample processing using solid phase extraction (SPE) was selected to demonstrate the ability of this technique to perform both extraction and concentration tasks. An Ascentis Express C18 column (5 cm × 4.5 mm, particle size: 5 μm) from Berlin (Germany) was utilized to obtain a fast HPLC analysis at 35 ºC. The mobile phase was water/acetonitrile (60:40, v/v) and it was delivered with a flow rate of 1 mL/min at room temperature and pressure was 3268 psi (225 bars) [20].
The system was calibrated with several standards and a response factor for BPA was generated for each detector. The pH values of aqueous solutions were measured with a Metrohm pH-meter (model: 827) supplied with a glass electrode. This allowed recovery data of the spiked sample to be calculated as µg/L.
Statistical analysis: The data was statistically analyzed for the significance difference was demonstrated at p ≤ 0.05 by using T-test and values were expressed as means ± S.E. Correlations of the changes in snails distribution with the other parameters were determined by Pearson’s correlation and stepwise multiple regression analysis. The significance level was set at P < 0.05.
Results
Results of physicochemical parameters were recorded at each site (Table 1). It was noticed that Giza sites have almost the same temperature, conductivity, DO, and pH. There were significant differences among Kafr El-Shiekh sites in conductivity and TDS, where, Qulin had the highest water conductivity (1302 µs/m) and TDS (901 mg/L).
A total of nine snail species were collected from the five sites of investigation during the study period (Table 2). The number of total specimens is 2711, 189, 323, 189, 4, 6, 18, 9 and 33 for B. alexandrina, Physa acuta, Planorbis planorbis, Lymnaea natalensis, Bulinus truncates, Bellamya unicolor, M. tuberculate, Helisoma duryi and Lanistes carinatus, respectively.
B. alexandrina snails were the most distributed species with 94.3% and 88.5% at Qulin and Kafr hakim, respectively. The results revealed that B. alexandrina represented 45.96% and 83.29% of all abundant snails in Giza and Kafr El Shiekh collected-sites, respectively.
The association pattern between B. alexandrina and other snail’s species is presented in (Table 2). The highest association was found between B. alexandrina and P. acuta followed by L. natalensis. However, B. alexandrina snails showed the lowest association with M. tuberculata, P. planorbis, and B. truncatus.
The frequency and relative coverage by the site of macrophytes are represented in (Table 3). During the survey study, five aquatic plant species; L. gibba, C. demersum, Eichhornia crassipes, Jussiae repen and Pistia stratiotes (Water lettuce) were observed. Results showed that L. gibba was the most represented aquatic plant with relative coverage 15, 13, 11, 0 and 0 in El Salmawy, Kafr Hakim, Qulin, Shinu and Kafr El-Sheikh, respectively. However, P. stratiotes confined presence in one site with total coverage 5.
In descending pattern, the most density of aquatic plants / site was recorded as 29, 22 and 15 at El-Salmawy, Kafr hakim, and Kafr El-Sheikh, respectively. Data revealed that L. gibba was the most predominant plant with B. alexandrina snails.
There is a positive correlation between the abundance of snails and the presence of aquatic plants. In Giza, the number of snails increased in the presence of L. gibba, C. demersum, and Pistia stratiotes. In Kafr El-Sheikh, the number of snails increased in the presence of L. gibba and J. repen.
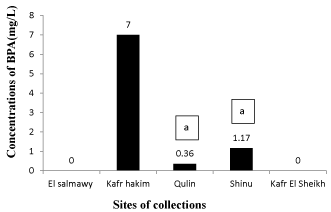
Determination of BPA was performed with a micellar mobile phase. An example of a chromatogram obtained under these conditions from a standard mixture of BPA (0.19 mg /mL). Chromatogram obtained from a standard solution of BPA showed the retention time (4.2 min). The recoveries of BPA were in the range of 98.3%-100%. The concentrations of BPA at Kafr Hakim, Qulin, and Shinu sites were 7.0, 0.36, and 1.17 mg/L, respectively (Figure 3).
Discussions
It is apparent that conditions in polluted field sites present a more complex picture than laboratory studies. This is not unexpected when one considers the numerous interactions occurring between biotic and abiotic factors. Nevertheless, a few generalizations are apparent [21].
The prevalence and diversity of snails could be increased or decreased depending on the class of pollutants. Under natural condition, snails are exposed to several environmental factors which produce a collective effect on the snails. In the present study, it was found that the highest percentage of total snail species (75.47 %) was collected at 28°C in Qulin, but when the temperature increased more than 34°C, the percentage was decreased to 5.86 % and 4.11% in Kafr El Sheikh and Shinu sites, respectively. The highest occurrence of B. alexandrina snails was recorded to be 94.3% and 88.5% at 28°C and 31°C, respectively. This is in coincidence with El-Khayat et al. [22], who revealed that snails can tolerate a wide range of temperature 19 – 34°C. However, it has been showed that snails can tolerate low temperature rather than a high temperature which can lethally affect them [23]. This indicates that snail species is highly sensitive to an elevation in temperature that may cause thermal stress on snail and also reduces the dissolved oxygen content of water body [24]. However, significant link was not found between snail abundance and water temperature [25].
The present study found that the highest percentage of B. alexandrina snail was collected under conductivity 1302 μS/m. This finding is in agreement with the previous study of Berrie [26], who promulgated that snails are not found in waters with low conductivity. This may be attributed to the ability of snails to tolerate a wide range of water hardness and these results were supported by [27], where the water with low hardness showed a reduction in the individual number and snail’s shells become relatively thin.
B. alexandrina was collected at TDS ranges of 120.1-901 mg/L. These results give an indication that the snails could survive under high concentrations of TDS. Our results could be confirmed by the observation of Hairson et al. [28], who reported that snails are not found in waters with low concentrations of TDS. Moreover, it has been postulated that certain snails found in habitat with higher salinity more suitable [29]. The snails can live in a wide range of mineral content in water till certain limiting [30]. On the contrary, it was found that the highest numbers of B. truncatus snails were found under low dissolved salts (100-300 ppm) while B. alexandrina snails were obtained from a range of 601-1000 ppm [31].
From the current work, it was found that the B. alexandrina species were collected at DO range of 2.65 – 2.71 mg/L. This result was almost within the range mentioned by [32], who found that the desired concentration of DO for snails ranged between 2.2 – 8.5 mg/L.
In the present work, B. alexandrina species were observed in almost the same range of water pH (7.22-7.33). it was stated that pulmonata snails Lymnaea sp., Bulinus sp. and B. alexandrina were collected from large as well as small canals in Behera Governorate and from narrow ditches, where pH ranged from 7.2 to 7.6 [33]. The present pH range found to be nearly similar to the observation by [34], who found that pH range was 7.2 – 10.9 for all the sites that harbored snails.
The present study has identified a total of nine snail species namely B. alexandrina; B. truncatus; L. natalensis; P. acuta; H. duryi; L. carinatus; B. unicolor; P. planorbis and M. tuberculata in five selected sites from two Egyptian governorates (Giza and Kafr El-Sheikh) during a survey period. This gives an indication of stable coexistence found only in habitats, which are capable of supporting mutually exclusive and conductive niches for different species population.
The present field survey detected that the B. alexandrina was the most enumeration of snail species. This may be attributed to that B. alexandrina was more tolerant than the other snail species to some of the examined parameters. This was in agreement with results of [23,35,36]. It was noted that B. alexandrina snails invested in smaller canals and drains. This notice is in accordance with Dazo et al. [37], who reported that B. truncatus was most abundant in large canals while B. alexandrina was most abundant in drains.
The highest percentage of total snail species (75.47 %) was collected at 28 °C, but when the temperature increased more than 34°C this percentage was decreased to 5.86 % and 4.11% in Kafr El-Sheikh and Shinu sites, respectively. However, B. alexandrina snails were recorded with the highest occurrence (94.3% and 88.5%) at 28 °C and 31 °C, respectively. B. alexandrina snail was found at TDS ranges from (120.1-901 mg/L) and dissolved oxygen range (2.65 – 2.71 mg/L).
The distribution of B. truncatus and B. alexandrina in two villages, El-Garda, and Salamoniya, in Menoufia Governorate was studied and differed greatly in the degree of chemical and fecal pollution of the watercourses [38]. This was probably due to the existence of the sewage disposal system in El-Garda village and its absence in Salamoniya. In spite of the high pollution of watercourses in Salamoniya, both Bulinus and Biomphalaria snails were found and were often infected. On the other hand, in El-Garda, in spite of the lower pollution of its watercourses, which would have been expected to be associated with higher snail counts, particularly in Kafr Tambidy canal which was less chemically polluted, B. truncatus was the only snail found and with very low counts.
The survey study observed five aquatic plant species; L. gibba, C. demersum, E. crassipes, J. repen and P. stratiotes where L. gibba and E. crassipes were the mostly infested aquatic plants. It has been showed that sites in which snails associated with macrophytes (64%) were characterized with higher ranges of chemicals, DO, and conductivity than that observed in sites with snails only indicating the helpful role of macrophytes for increasing snail tolerance to unfavorable conditions [22]. Moreover, a significant association between vegetation density and snail occurrence was found [39]. In addition, it has been reported that in general, adverse effects of water pollution on snail biology were modified by biotic factors including food supplies, aquatic plants, behavioral and physiological adaptation [40].
In the current study, there is a positive correlation between the abundance of snails and the presence of aquatic plants. We observed an association between moderate density of the recorded plants especially L. gibba and B. alexandrina in most sites. This is in agreement with findings of De souza and De melo [41,42], that considered the aquatic vegetation (such as Eichhornia sp. and Lemna sp.) consequently, providing shelter and food resource for the snails. Moreover, it has been found that E. crassipes and L. gibba were positively correlated with B. truncatus and B. alexandrina, respectively [19]. The reasons could be because the snails depend directly or indirect on the aquatic plant, where they cannot live or reproduce without aquatic vegetation. They prefer to deposit their egg masses on the plant materials as well as on hard and broad leaves [43].
Environmental degradation information is factored into estimates of exposure. The environmental degradation of polycarbonate grade BPA, used in the manufacture of plastics, was measured using waters samples from the five sites. The concentrations of BPA in Kafr Hakim, Qulin, and Shinu sites were 7.0, 0.36, and 1.17 mg/L, respectively. This variation in BPA concentration could be illustrated as surface-water concentrations of BPA vary considerably depending on the location, sampling period, and how the results are reported [44]. It was found that BPA concentrations from monthly samples on multiple rivers ranged 0.07-4.0 µg /L [45]. However, studies the levels of BPA in landfill leachate in Japan reported that the concentrations range and 1.3-17,200 µg/L [46].
In conclusion, the most important of the associating vegetation was L. gibba which correlated with B. alexandrina and could be served as an indicator plant for the snail in selected sites.
- Kumari PR (2013) Detergent induced protein alterations in freshwater gastropod Bellamya bengalensis (Lamarck). Indian J Sci Res 4: 57-60. Link: https://goo.gl/reJlUx
- Hernandez-Rodriguez G, Zumbado M, Luzardo OP, Monterde JG, Blanco A, et al. (2007) Multigenerational study of the hepatic effects exerted by the consumption of Haniokanonylphenol and 4-octylphenol contaminated drinking water in Sprague-Dawley rats. Environ Toxicol Pharmacol 23: 73-81. Link: https://goo.gl/Gtctu0
- Oehlmann J, Schulte-Oehlmann U, Bachmann J, Oetken M, Lutz I, et al. (2006) Bisphenol A induces super-feminization in the ramshorn snail Marisa cornuarietis (Gastropoda: Prosobranchia) at environmentally relevant concentrations. Environ Health Perspect 114: 127-133. Link: https://goo.gl/bPfQOS
- Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, et al. (2007) An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol 24: 225-239. Link: https://goo.gl/dIRqrx
- Funakoshi G, Kasuya S (2009) Influence of an estuary dam on the dynamics of bisphenol A and alkylphenols. Chemosphere 75: 491-497. Link: https://goo.gl/gDWgnn
- Bolsunovskii AIA, Ermakov AI, Burger M, Degermendhzi AG, Sobolev AI (2002) Accumulation of industrial radionuclides by the Yenisei river aquatic plants in the area affected by the activity of the mining and chemical plant. Radiats Biol Radioecol 42: 194-199. Link: https://goo.gl/gQaqe3
- Thorp JH, Thorp and Rogers DC (2014) Covich's Freshwater Invertebrates: Ecology and General Biology. 4th edition. Elsevier. Link: https://goo.gl/MXHs6v
- Dillon RT (2004) The Ecology of Freshwater Molluscs. 1st edtion. Cambridge university press. Link: https://goo.gl/acDNve
- Underwood GJC (1991) Growth enhancement of the macrophytes Ceratophyllum demersum in the presence of the snail Planorbis planorbis: the effect of grazing and chemical conditioning. Fresh Water Biol 26: 325–334. Link: https://goo.gl/G9mcix
- Underwood GJC, Baker JH (1991) The effect of various aquatic bacteria on the growth and senescence of duckweed (Lemna minor). J Appl Bacteriol 70: 92-196. Link: https://goo.gl/ZVpqlM
- Madsen H (1992) Food selection by freshwater snails in the Gezira irrigation channels, Sudan. Hydrobiol 228: 203-217. Link: https://goo.gl/953S3S
- Habdija I, Latjner J, Belinic I (1995) The contribution of gastropod biomass in macrobenthic communities of a karstic river. Inter Rev Hydrobiol 80: 103-110. Link: https://goo.gl/MjapA9
- Freitas JR, Santos MBL (1995) Current advances in the study of snail-snail interactions, with special emphasis on competition process. Mem Inst Oswaldo Cruz 90: 261-269. Link: https://goo.gl/WvNtzo
- Mkoji GM, Mungai BN, Koech DK, Hofkin BV, Loker ES, et al. (1992) Does the snail Melanoides tuberculata have a role in biological control of Biomphalaria Pfeiffei and other medically important African pulmonates? Ann Trop Med Parasitol 86: 201-204. Link: https://goo.gl/96KR54
- Giovanelli A, da Silva CLPAC, Leal GBE, Baptista DF (2005) Habitat preference of freshwater snails in relation to environmental factors and the presence of the competitor snail Melanoides tuberculatus (Müller, 1774). Mem Inst Oswaldo Cruz 100: 169-176. Link: https://goo.gl/FS8XZj
- Abou-El-Naga IF (2013) Biomphalaria alexandrina in Egypt: Past, present and future. J Biosci 38: 665–672. Link: https://goo.gl/UIgfhJ
- Yousif F, Khalil M, El-Emam M (1992) Evaluation of three common tools in estimating Biomphalaria alexandarina population in irrigation ditches. Egypt J Bilh 14: 151-158. Link: https://goo.gl/zuOlmp
- Yousif F, El-Emam M, Abdel Kader A, Sharaf El-Din A, El-Hommossany K, et al. (1998) Schistosomiasis in newly reclaimed areas in Egypt. 1. Distribution and population seasonal fluctuation of intermediate host snails. J Egypt Soc Parasitol 28: 915-928. Link: https://goo.gl/HCms4K
- Hussein MA, Obuid-Allah AH, Mahmoud AA, Fangary HM (2011) Population dynamics of freshwater snails (Mollusca: Gastropoda) at Qena Governorate, Upper Egypt. Egypt Acad J Biol Sci 3: 11-22. Link: https://goo.gl/ymjjMa
- Sohrabi R, Bahramifar N, Javadian H, Agarwal S, Gupta VK (2016) Pre-concentration of a trace amount of Bisphenol A in water samples by palm leaf ash and determination with high-performance liquid chromatography. Biomed Chromatogr 30: 1256-1262. Link: https://goo.gl/AiX37G
- Morley NJ (2010) Interactive effects of infectious diseases and pollution in aquatic molluscs. Aquat Toxicol 96: 27-36. Link: https://goo.gl/WPz4jJ
- El-Khayat, HM, Mostafa BB, Mahmoud KMA, El-Said KM, Ismail NMM (2009) The association between freshwater snails, macrophytes and water quality in different water courses in Egypt. New Egypt J Med 40: 381-392.
- Mahmoud KA (1994) The feeding ecology of the snail intermediate hosts of schistosomiasis in Egypt. M.Sc. Thesis, Faculty of science, Cairo University.
- Hofkin BV, Mkoji GM, Koech DK, Loker ES (1991) Control of schistosome-transmitting snails in Kenya by the North American crayfish Procambarus clarkii. Am J Trop Med Hyg 45: 339-344. Link: https://goo.gl/heOtwG
- Kariuki HC, Clennon JA, Brady MS, Kitron U, Sturrock RF, et al. (2004) Distribution patterns and cercarial shedding of Bulinus nasutus and other snail species in the Msambweni area, Coast Province, Kenya. Am Trop Med Hyg 70: 449-456. Link: https://goo.gl/T8hOui
- Berrie AD (1970) Snail problems in Africa schistosomiasis. Adv Parasitol 8: 43-96. Link: https://goo.gl/BbaCw6
- Abdel-Malek E (1958) Factors conditioning the habitat of bilharziasis intermediate hosts of the family planorbidae. Bull World Health Organ 18: 785-818. Link: https://goo.gl/BpJhNk
- Hairson NG, Hubenick B, Watson JM, Oliver LJ (1958) An evaluation of techniques used in establishing snail population. Bull World Health Organiz 19: 661-672. Link: https://goo.gl/q4i8II
- Ofoezie IE (1999) Distribution of freshwater snails in the man-made Oyan Reservoir, Ogun State, Nigeria. Hydrobiol 416: 181-191. Link: https://goo.gl/JlFtbq
- Didonato GT, Summers JK, Roush TH (2003) Assessing the ecological condition of a coastal plain watershed using a probabilistic survey design. Environ Monit Assess 85: 1-21. Link: https://goo.gl/QuMLCU
- Saad AEA, Mostafa BB, El-Magd SSA, Azzam AMA (2012) Impact of some environmental factors on the distribution of certain vector snails in five Egyptian Governorates. Egypt J Aquat Biol Fish 16: 33-40. Link: https://goo.gl/Sp1hV1
- Njoku-Tony, RF (2011) Effect of some physicochemical parameters on the abundance of intermediate snails of animal trematodes in Imo state, Nigeria. Res 3: 5-12. Link: https://goo.gl/yyF1AH
- Ashmawy K, Abu-El-Wafa SA, El-Bahi MM, Diab MR (1994) Incidence and ecology of freshwater snails in Beheira province. Assiut J Vet Med 30: 101-113.
- Ntonifor HN, Ajayi JA (2007) Studies on the ecology and distribution of some medically important freshwater snail species in Bauchi state, Nigeria. Int J Biol Chem Sci 1: 121-127. Link: https://goo.gl/16vI9N
- Habib MRA (2010) Studies on the effect of the geographical distribution of Biomphalaria alexandrina snails on their susceptibility to Schistosoma mansoni infection in some localities in Egypt. M.Sc. Thesis, Fac. Sci., Menoufia. Univ., Egypt.
- El-Khayat HMM, Mahmoud KM, Mostafa BB, El-Deeb FA, Tantawy AA, et al. (2011) Habitat characteristics for different freshwater snail species as determined biologically through macroinvertebrate information. Egypt Soc Parasitol 41: 651-664. Link: https://goo.gl/JMC57u
- Dazo BC, Hairston NG, Dawood IK (1966) The ecology of Bulinus truncatus and Biomphalaria alexandrina and its implications for the control of Bilharziasis in the Egypt -49 Project Area. Bull World Health Organiz 35: 339-356. Link: https://goo.gl/ykGZ4u
- Khairy AEM (1998) Water contact activities and schistosomiasis infection in Menoufia, Nile Delta. East Mediterr Health J 4: 100-106. Link: https://goo.gl/BCdNzr
- Kloos H, Jannotti Passos L K, Loverde P, Oliveira RC, Gazzinelli A (2004) Distribution and Schistosoma mansoni infection of Biomphalaria glabrata in different habitats in a rural area in the Jequitinhonha Valley, Minas Gerais, Brazil: environmental and epidemiological aspects. Mem Inst Oswaldo Cruz 99: 673-681. Link: https://goo.gl/6mdpgO
- Mahmoud AAF (2001) The schistosomes and their intermediate hosts. In: Schistosomiasis.
- Pasvol, G, Hofffman, S.L (eds). Imperial College press, London, pp. 7-84.
- De souza MAA, De melo, AL (2012) Ecological aspects of Biomphalaria in endemic areas for Schistosomiasis in Brazil. Schistosomiasis. Link: https://goo.gl/3WrbRu
- Van Schayck CP (1985) Laboratory studies on the relation between aquatic vegetation and the presence of two bilharzia bearing snail species. J Aquat Plant Manag 23: 87-91. Link: https://goo.gl/weI37f
- Flint S, Markle T, Thompson S, Wallace E (2012) Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manage 104: 19-34. Link: https://goo.gl/GHk7Zx
- Azevedo DA, Lacort C, Barceló, B (2001) Occurrence of Nonylphenol and Bisphenol-A in Surface Waters from Portugal. J Braz Chem Soc 12: 532–537. Link: https://goo.gl/uEoBM1
- Yamamoto TA, Yasuhara A, Shiraishi H, Nakasugi O (2001) Bisphenol-A in hazardous landfill leachates. Chemosphere 42: 415-418. Link: https://goo.gl/rLyfVU
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
