International Journal of Veterinary Science and Research
Effect of a Beneficial Flora Colonization of Pen Surfaces on Health of Weanling Piglets
Eric Royer1*, Julia Plateau-Gonthier2*, Eric Chevaux2 and Fernando Bravo de Laguna Ortega2
1Wageningen University & Research, 6700 AH Wageningen, Netherlands
2Lallemand SAS, 31702 Blagnac, France
Julia Plateau-Gonthier, Lallemand SAS, 31702 Blagnac, France, E-mail: [email protected]
Cite this as
Royer E, Plateau-Gonthier J, Chevaux E, de Laguna Ortega FB. Effect of a Beneficial Flora Colonization of Pen Surfaces on Health of Weanling Piglets. Int J Vet Sci Res. 2024;10(3):063-069. Available from: 10.17352/ijvsr.000150Copyright License
© 2024 Royer E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Undesirable bacterial colonization of farm surfaces affects animal health after weaning. The objective of the study was to test the preventive effects of a positive biofilm formation on the surfaces of piglet facilities on beneficial flora colonization and animal health.
Methods: 494 piglets from two weaning batches (Exp.1 and Exp.2) were allocated in 2 identical rooms. The rooms were sprayed either with water (Control) or a mix of selected bacteria strains (LFP) 48 h before the entrance, and again on day 15 in Exp.1, and on days 5, 12, 19, 26, and 33 in Exp.2. The microbiological status of the surfaces was assessed on days 0, 5, and 14 and on days -2, 0, 5, 7, and 35 in Exp.1 and 2, using peptone water swabs. Fecal consistency was scored on days 5, 8, 14, 21, and 28 on 16 randomly selected piglets per treatment. Statistical analysis was performed in SAS 9.4. Non-parametric tests were used to analyze the microbiological data, fecal scores, and death distributions.
Results: There was a significant (P < 0.05) higher load of aerobic bacteria (Lactobacillus spp., Bacillus spp.) in LFP pen surfaces in both experiments. Fecal scores were significantly improved on day 8 in Exp.1 (P < 0.01) and on days 9 (P = 0.01) and 28 (P < 0.01) in Exp.2. Digestive disease outbreaks occurred 2 days later in Exp.1 and 7 days later in Exp.2 in LFP rooms.
1Conclusion: spraying a beneficial flora on surfaces may result in a protective positive biofilm that would help piglets to better deal with the weaning challenges.
Abbreviations
C&D: Cleaning and Disinfection; SD: Standard Deviation; Exp: Experiment; LFP: Lalfilm Pro; CON: Control; NE: Net Energy; NDF: Neutral Detergent Fiber; ADF: Acid Detergent Fiber; ADL: Acid Detergent Lignin; CFU: Colony Forming Units; LOD: Limit of Detection; IU: International Units; BW: Body Weight; PNDAR: National Program for Agricultural Development; EAAP: European Association for Animal Production
Introduction
Hygiene and biosecurity are among the main keys to reducing the use of antibiotics in animal farming. Continuously, new methods for Cleaning and Disinfection (C&D) have been evaluated for their efficiency, water use, and cost to disrupt and neutralize undesirable biofilms [1-3]. However, achieving sufficient reduction in levels of Enterobacteriaceae by C&D procedures may be difficult [4, 5]. In this context, an emerging preventive method is based on the post-disinfection inoculation of the environment by beneficial bacteria to promote a positive microbial environment during the first days of the production cycle. A biofilm is a microbial community attached to a surface. It has properties conferring many advantages to bacteria, and its level of organization is higher than that of single cells [6]. Biofilm formation is an issue in many industries, such as the food industry [7], or farming, where bacterial species with biofilm-forming ability can attach and persist in equipment [8], surfaces [9], or eggs [10], becoming a source of disease or animal products contamination.
The preventive application of a cocktail of beneficial bacteria on pig farms´ surfaces aims to create a positive biofilm that limits the growth of potential pathogens on the treated surfaces. [11] used a bacterial complex (lactic acid bacteria and Bacillus subtilis) on the surfaces of farrowing and post-weaning rooms and evaluated the impact on digestive health and growth [12]. This complex was applied on the surfaces after C&D and was repeated regularly during the life cycle of the animals. The application promoted better digestive health (assessed by fecal score) during the suckling and post-weaning periods. Nevertheless, the effect on animals is still to be documented and may have some limitations since the product is not directly fed to the pigs. Therefore, expected short-term effects may be more related to the absence of disease outbreaks or overall health improvement instead of performance improvement.
The aims of the present study were to document: 1) the potential of the application of a positive and protective flora based on Bacillus spp. and lactic acid bacteria to secure the microbial profile of farm surfaces, and 2) the risk decrease in health issues due to the growth mitigation of harmful microorganisms in the farm environment.
Materials and methods
The study was performed under field conditions using the herd of the IFIP experimental farm of Romillé (France) approved for animal experimentation (nº 35-245-28). Pigs were humanely cared for under the supervision of the herd veterinarian. As housing was adequate and the study did not involve any specific constraint for animals, no specific submission was required except following guidelines for animal care edited by the French Ministry of Agriculture.
A total of 494 (Large White × Pietrain) × (Large White × Landrace) female and castrated piglets from two subsequent batches were weaned at 28 ± 1 days (mean ± SD) of age, 8.42 ± 1.78 kg and 8.67 ± 1.68 kg in batch 1 and 2, respectively, and used in two experiments (270 piglets in Exp.1 and 224 piglets in Exp.2). In each experiment, the animals were randomly allotted according to sex, body weight, and litter origin to one of the two experimental treatments: 1. Control (CON); 2. Lalfilm Pro (LFP), each treatment corresponding to a room of 14 single-sex pens of 7-10 piglets. As diarrhea of suckling piglets was observed for 14 of the 23 litters of Exp.1, this criterion was added to randomization so that pens had the same number of piglets coming from litters suffering diarrhea.
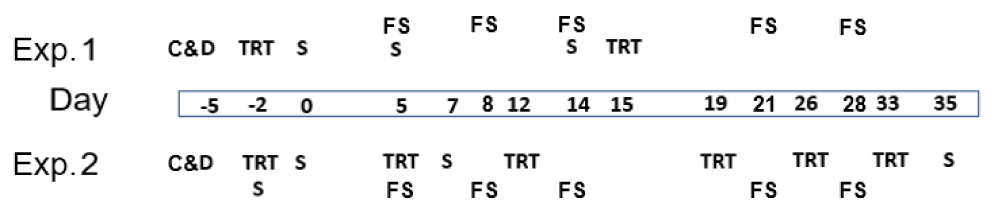
After emptying the previous batch, rooms were cleaned with high-pressure water and detergent (non-ionic surfactants, quaternary ammoniums, sodium hydroxide; Lipoclean, Farm’Apro, Plestan, France), and disinfection (Sanifarm NF, Farm’Apro, Plestan, France) was carried on day -5 before the entrance of the piglets (Table 1). On day 0, the LFP room was sprayed with a highly concentrated mix of live Bacillus spp. and lactic acid bacteria (Lalfilm Pro, Lallemand SAS, Blagnac, France) with a backpack sprayer at a dose of 10 g/100 m2 deployed surface, to target a minimum concentration of 2 × 109 colony forming units (CFU)/m2 of deployed surface. Each pen had front and side plastic partitioning panels, a back brick wall, a metal feeder, and a plastic slatted floor. Each room had a calculated deployed surface of 230 m2, therefore, 23 g of the product was diluted in 5 l of tepid tap water without any additional treatment and transferred to the sprayer. The total water sprayed in each room was 7.6 l according to the supplier´s recommendations. The CON rooms were sprayed with the same amount of water. In Exp.1 the rooms were sprayed again 15 days after the entrance of the animals, whereas in Exp.2 the rooms were sprayed again on days 5, 12, 19, 26, and 33 after the entrance. Additionally, in Exp.2 the CON and LFP rooms were switched to avoid any room effect or cross-contamination.
In Exp.1, the C&D was performed with only a partial emptying of the manure from the pits below the slatted floor in both rooms, whereas in Exp.2, C&D included a total emptying and disinfection of the gutters. Animal density in the experiments was between 0.31 and 0.44 m2/piglet. In Exp.2 density was increased in order to increase the stress and microbial pressure. The animals followed a two-phase feeding program: between 0 and 14 days, and between 15 and 35 days, when both experiments finished, with identical diets in both experiments. Piglets were given free access to feed and water from stainless steel dry meal hoppers and a drinking bowl per pen. All diets (Cooperl Coop., Vitré, France; Table 1), met or exceeded the nutritional recommendations issued by IFIP-Institut du Porc [13], in particular concerning the digestible lysine (Lysd) to net energy (NE) ratio and the balance between the amino acids (10.6 MJ NE per kg, 13.7 g Lysd per kg as weight). However, to not interfere with the inflammation process of the animal, in phase 1 the digestible tryptophane to Lysd ratio was 19%.
Air temperature (from 27 to 24 °C during the experiments), ventilation, and lightning (from 09:00 to 18:00) were identical in both rooms. To avoid microbiological cross-contamination between the two rooms, special care of biosecurity was managed during the study, making it necessary to change the overall suit and boots to enter the rooms. For weighing and fecal scoring, instruments were cleaned between each pen and washed and disinfected between each room.
The microbiological status of the farm surfaces was assessed using peptone water swabs (Ref 4122A, Sodibox, Nevez, France). Based on the outcomes of Exp.1, the sampling design was modified in Exp.2 by increasing the number of sampling days and by dropping the identification of certain bacterial species and the number of pens. One 32 × 17 cm swab was used to sample each of the 8 central pens of each room in Exp.1, and each of the 4 central pens in each room in Exp.2. In Exp.1, sampling was on days 0, 5, and 14, while in Exp.2 sampling was on days -2, 0, 5, 7, and 35 (Table 2). The rationale behind the sampling points was as follows: day -2, before application of either water or LFP, in order to check the efficiency of the C&D process; day 0, after application and before the entrance of the animals, to check the presence of the beneficial flora on pen surfaces; samples of the rest of the days were used to check the evolution of the different target microbial species. The wiped surface was 1 m2 per pen, from both plastic separation panels. At each timepoint, a different spot was wiped to not be affected by the previous sampling. After each sampling, swabs were immediately picked up and transferred under refrigerated transport at 4°C conditions to Labocea Laboratory (Fougères, France) to be assessed for Enterobacteriaceae (NF V 08-054), lactic acid flora (Internal method MRS 48 h 44°C), coliforms 44°C (NF V 08-060), coagulase-positive Staphylococci (NF EN ISO 6888-2 V 08-014-2), intestinal Enterococci (internal method Slanetz-BEA), and spores of aerobic bacteria 30°C (internal method PCA TT 10 min 80°C) in Exp.1. For Exp.2, samples were assessed for coliforms 44°C, intestinal Enterococci, and spores of aerobic bacteria 30°C (Table 1).
Health and feeding or behavioral changes of the animals were daily monitored and all observed symptoms of disease, any veterinary treatment, or death occurring during the experimental period were recorded. The fecal consistency was individually scored by a trained person at 10:00 on days 5, 8, 14, 21, and 28 in both experiments on sixteen previously and randomly selected piglets per treatment from eight pens by treatment (two piglets by pen), using a continuous scale of 5 levels where 1 = hard and dry feces; 2 = well-formed firm; 3 = soft, moist stool; 4 = loose semi-liquid feces, and 5 = watery feces [14]. The same piglets were scored at each observation. A scheme of the sampling points is shown in Figure 1.
Statistical analysis was performed using the procedures of SAS (SAS 9.4, SAS Inst., Cary, NC, USA). Non-parametric tests were used to analyze the results of bacterial measurements, fecal scores, and death distributions. The two-sample Wilcoxon test was used to compare the treatments. Results of flora enumeration are medians in CFU/m2 for 8 (Exp.1) or 4 pens (Exp.2) per treatment. For calculations, results were reported as equal to the limit of detection (LOD) for concentrations lower than LOD and as a higher quantified value for concentrations exceeding LOD. All the probability values (P-values) lower than 0.05 were considered to be significant, and between 0.05 and 0.1 were considered as a trend.
Results
All pens were free of contamination on day 0 in Exp.1 or on days -2 and 0 in Exp.2 (Table 3). The concentration of spores of aerobic bacteria was always higher in the LFP pens than in the CON pens in both experiments (P < 0.05; Table 3). On day 5 of Exp.1, spores of aerobic bacteria were significantly higher for LFP pens than for CON ones (P < 0.05) and lactic flora count was significantly lower for LFP (P < 0.01; Table 3). In Exp.1, Enterobacteriaceae counts tended to be higher in the CON pens compared to the LFP pens on day 5 (P < 0.10; Table 3), as these bacteria could be detected in six of the eight CON pens and in only two of the eight CON pens (data not shown). Similarly, coliforms at 44 °C were numerically higher on day 5 in CON pens compared to LFP pens in both trials. By contrast, intestinal Enterococci were numerically higher on day 5 in the LFP pens in Exp.2, however, differences in coliforms at 44°C and intestinal Enterococci were not significant. On day 35 in Exp.2, coliforms tended to be lower in the CON pens compared to the LFP pens (P = 0.10; Table 3).
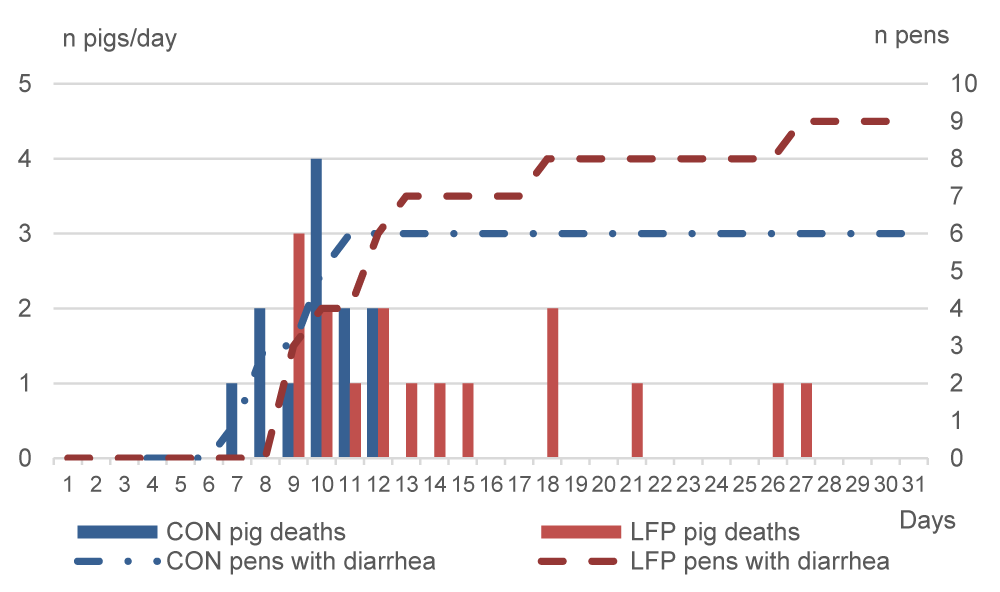
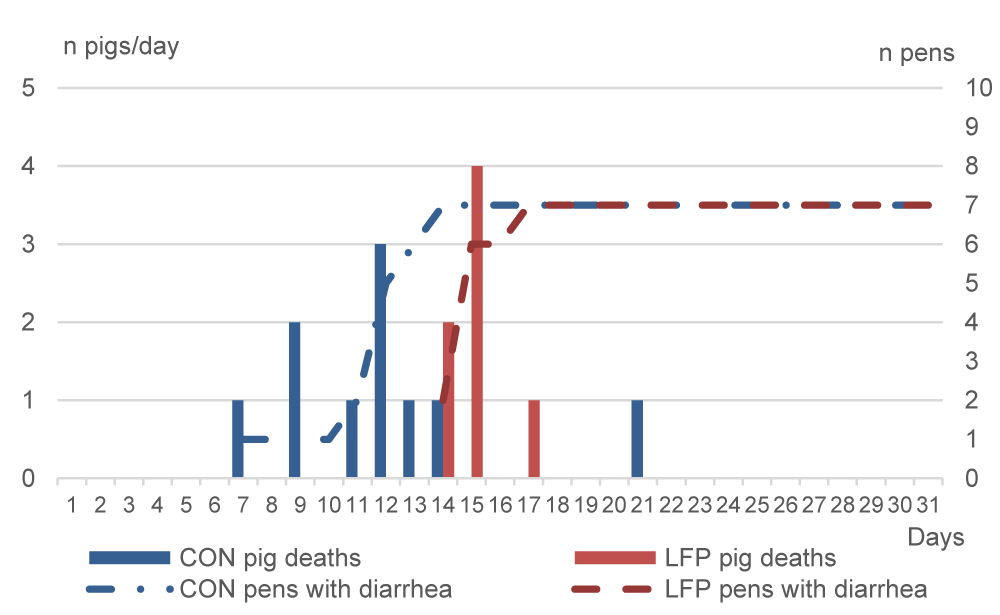
As anticipated from the moderate cases of diarrhea in the months preceding the study, both trials had sanitary events: diarrhea and digestive pathologies. The total number of dead or culled piglets averaged 11% in Exp.1 and 10% in Exp.2. For Exp.1, an outbreak of diarrhea occurred on days 7 and 8 only in the CON room resulting in the death from dehydration, or enteritis, or bacteremia, or all, of three piglets, whereas diarrhea was not observed in the LFP piglets (Figure 2). Therefore, 100000 IU/kg BW of colistin was administered from day 8 for five consecutive days via water to all piglets in the CON room. The diarrhea outbreak occurred two days later in the LFP room, and three piglets died on day 9 (Figure 2). On day 10, diarrhea was largely spread in the LFP room, therefore the complete room was also treated for five days. Additionally, seven CON and eight LFP very dehydrated piglets were individually administered one injection of 10 mg ampicillin and 25,000 UI colistin/kg body weight (BW) on days 10 or 11. Therefore, all CON piglets received colistin between days 8 and 12, and all LFP piglets between days 10 and 14. In Exp.2, diarrhea occurred from day 7 in the CON room, where a total of nine piglets died between days 7 and 14 (Figure 3). Seven days later, on day 14, the diarrhea appeared also in the LFP room and seven LFP piglets died between days 14 and 17. CON piglets of the affected pens were treated on day 7 with injections of 50000 IU of colistin and 0.02 g ampicillin per kg BW for three consecutive days from day 7 to 9. Another CON pen had to be treated between days 11 and 13. Two LFP pens were treated between days 15 to 17 and one pen between days 15 and 16. Additionally, two pigs of one LFP pen were treated on days 15 and 16, and the whole pen on day 17.
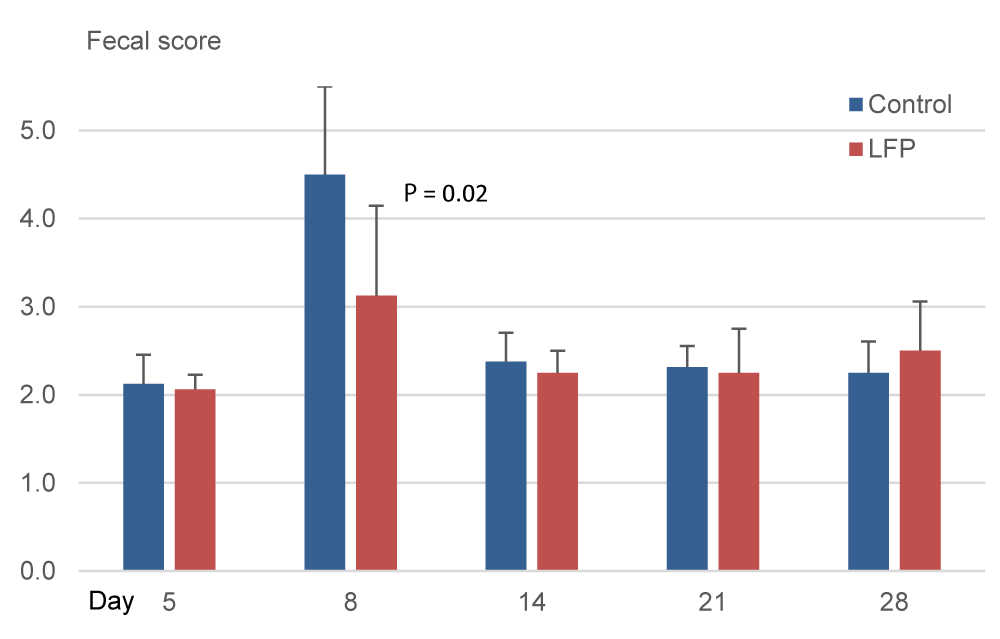
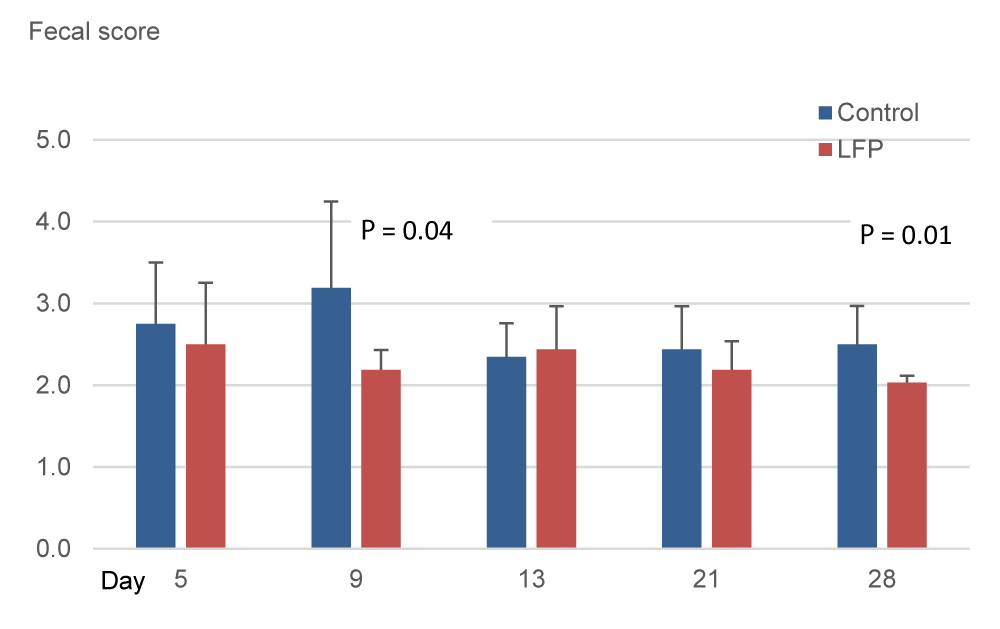
In Exp.1, measurements indicated a regular fecal consistency on day 5. Three days later, the scores increased resulting in a significantly higher mean score on day 8 for CON treatment compared to LFP treatment (P < 0.05; Figure 4). Additionally, a significantly higher number of piglets per pen with watery feces (score≥3.5) was measured for the CON treatment compared to the LFP treatment (1.75 vs 0.75 out of 2 piglets/pen, i.e., 81% vs 37%; P < 0.05; data not shown). In Exp.2, the scores were significantly higher in the CON piglets compared to the LFP piglets on day 9 (P < 0.05: Figure 4) and on day 28 (P < 0.05; Figure 5).
Discussion
This study evaluated how the application of a blend of Bacillus spp. and lactic acid bacteria may influence the surface flora of pens and the health of weaned piglets raised under field sanitary conditions. The absence of microbial contamination on day 0 in Exp. 1 and days -2 and 0 in Exp. 2 suggests the efficacy of the C&D processes. Biofilms produced by different bacterial species have a high degree of similarity [15], indeed, a biofilm could be formed by a consortium of bacteria [16]. If that is the case, the metabolism of the biofilm may vary compared to a mono-specie biofilm. For that reason, a low microbial load from previous production batches at the moment of the LFP application is important for the targeted biofilm development. In this context, the bacteriological results in both experiments indicated an adequate colonization of surfaces by Bacillus spp. from LFP following the C&D process. Therefore, it seems feasible to spray and establish a positive flora of the room surfaces before piglets enter the post-weaning house, in agreement with previous results in chicken buildings [17].
Weaning stress directly impacts the gastrointestinal tract structure and function of piglets [18, 19]. During this time, opportunistic pathogens such as Enterobacteriaceae and coliforms can proliferate, causing diarrhea and death due to dehydration, enteritis, bacteremia, or them all. On day 5 of Exp.1, Enterobacteriaceae were detected in fewer LFP pens than CON pens, and surfaces of CON pens tended to have higher counts of Enterobacteriaceae. Regarding coliforms, both trials were consistent on day 5 and day 7, with non-significant but numerically higher counts in CON pens compared to LFP pens. In our study, as a result of the LFP treatment of the rooms, disease outbreaks occurred two days later in Exp.1 and seven days later in Exp.2 compared to the CON rooms. The consistent delay in the appearance of diarrhea could be related to the higher presence of those potential pathogens in the CON pens. However, this finding deserves further investigation. Besides that, LFP application had an impact on fecal consistency, and mean scores were significantly improved on day 8 in Exp.1 and days 9 and 28 in Exp.2, which agrees with the transitory hardening of feces reported by [12]. We can further hypothesize that the LFP treatment affected the surface flora of pens and that this modification could explain the delay in the first occurrence of post-weaning diarrhea and fecal score. The bigger gap in the appearance of diarrhea in Exp.2 compared to Exp.1, may be explained by the application schedule applied, with two applications in Exp.1 and six applications in Exp.2. A re-application may renew the presence of beneficial flora, conferring extra benefits to the piglets. Another reason could be the improvement in Exp.2 due to the higher stocking density in the pens since a higher density implies lower performances and impaired immunity [20].
Unexpectedly, Enterobacteriaceae and coliforms could not be detected on day 14 in Exp.1, neither in CON nor in LFP pens. The explanation could be in the antibiotic treatment applied to all the animals between days 8 and 12 to control the diarrhea outbreak, which could have reduced the spread of the bacteria [21], and disseminated colistin in the pens [22, 23]. In Exp.2, similar evolutions of the surface microflora were seen from day 7 to 35 among CON pens and likewise among LFP pens, which may indicate the stabilization of the microbial environment with time post-weaning. Previous studies established that the digestive flora may not be stabilized before 10 days after weaning [24]. Indeed, the development of the intestinal microbiota in the piglet is a gradual and sequential process, that relates to non-dietary and dietary factors [25]. Therefore, piglets that accumulate a higher diversity of potentially beneficial microbes before and during the high-risk weaning period may have a competitive advantage [26]. This proposition supports strategies promoting pig health through gut microbiota engineering [27]. Indeed, the composition of the flora is influenced by the hygienic status of the farm and by the introduction of solid feed [28]. Moreover, ileal and colonic bacterial communities of weaning piglets are influenced by the dietary addition of prebiotics or probiotics [29].
In the present study, deaths of diseased piglets were probably associated with Escherichia coli diarrhea as a K88 strain, as well as Clostridium perfringens, were isolated after autopsies on two dead piglets on day 15 of Exp.2. Both piglets had hypertrophy of mesenteric lymph nodes. The same strain of K88 had been historically diagnosed in the herd according to the responsible person in the experimental center. Initially, on day 5, only a non-hemolytic Escherichia coli was found in the liquid feces of CON and LFP piglets. According to the typical pathogenesis [30, 31], the disease outbreaks in our study are likely the result of weaning stress resulting in undernutrition, and pathogen survival in the environment, quickly enhanced by a multifactorial infection and leading to mortality caused by Escherichia coli.
The differences in gut health observed in our study seem to be driven by microbial environment differences in the CON and LFP rooms, which may have affected the gut microbiota of the piglets of those rooms. Gut microbiota can certainly affect piglet gut health [32]. By nutritional means, it seems simpler and more direct to modulate gut microbiota than by environmental interventions. Piglets acquire the environmental microbiota by exploring and leaking, but the process is less direct than through the diet. In humans, it is reported that people exposed to swine farms cause a difference in the proportion of some bacteria compared to non-exposed people [30]. Therefore, we can hypothesize that a constant application of beneficial flora in the farm surfaces batch after batch may change the farm microbial environment in the long term, thus improving the overall health of the farm along batches.
Conclusion
As a conclusion, our results support the potential of early environmental microbiota modulation as a tool for reducing susceptibility to post-weaning diarrhea. The study suggests that the dominant aerobic bacteria present in a concentrated blend of live Bacillus spp. and lactic acid bacteria limited the growth of undesirable bacteria on pen surfaces leading to a mitigation of early post-weaning diarrhea. Therefore, by allowing the set-up of a safer microbial environment at weaning, the application of this protective flora may be a promising complementary action to the C&D process in its contribution to limiting diarrhea outbreaks.
It is recommended to study the long-term effect of LFP application on farm surfaces on animal health and performance. Since two different methods were used in the study, it should be determined in a future study which methodology optimizes the results.
The trials were supported in part by Lallemand SAS (Blagnac, France) and by the French National Program for Agricultural Development (PNDAR). The technical assistance of E. Gault, D. Loiseau, R. Richard IFIP-Institut du porc) and L. Saulnais, J.P. Commereuc, (Station Expérimentale Romillé) is gratefully acknowledged. We also like to thank all the people of Lallemand Animal Nutrition, IFIP-Institut du porc, Labocea, and Bio Chene-Vert for aiding in the design of this study or in sample collection and processing.
- Corrégé I, Azevedo Araujo C de, Le Roux A. Development of a method to assess the efficiency of on-farm cleaning and disinfection. Journées Rech Porcine. 2003;35:419-426.
- Corrégé I, Dubroca S. Efficiency and cost comparison of different cleaning and disinfecting processes for pig farms. In: In between congress of the ISAH. 2004;285-286. Available from: https://www.isah-soc.org/userfiles/downloads/symposiums/2004/Correge.pdf
- Misra S, Van Middelaar CE, Jordan K, Upton J, Quinn AJ, de Boer IJM, et al. Effect of different cleaning procedures on water use and bacterial levels in weaner pig pens. PLoS ONE. 2020;15(11):e0242495. Available from: https://doi.org/10.1371/journal.pone.0242495
- Mannion C, Leonard FC, Lynch PB, Egan J. Efficacy of cleaning and disinfection on pig farms in Ireland. Vet Rec. 2007;161:371–375.
Available from: https://doi.org/10.1136/vr.161.11.371. - Corrégé I, Hémonic A. Influence of the cleaning and disinfection process for pig farms on the efficiency of decontamination and the persistence of Salmonella. Journées Rech Porcine. 2012;44:97-98. Available from: https://www.journees-recherche-porcine.com/texte/2012/sante/PS4f.pdf
- Flemming HC, Wuertz S. Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Microbiol. 2019;17:247-260.
Available from: https://doi.org/10.1038/s41579-019-0158-9. - Van Houdt R, Michiels CW. Biofilm formation and the food industry, a focus on the bacterial outer surface. J Appl Microbiol. 2010;109(4):1117–1131. Available from: https://doi.org/10.1111/j.1365-2672.2010.04756.x
- Latorre AA, Van Kessel JS, Karns JS, Zurakowski MJ, Pradhan AK, Schukken YH, et al. Biofilm in milking equipment on a dairy farm as a potential source of bulk tank milk contamination with Listeria monocytogenes. J Dairy Sci. 2010;93(6):2792-2802.
Available from: https://doi.org/10.3168/jds.2009-2717 - Tassinari E, Duffy G, Bawn M, Burgess CM, McCabe EM, Lawlor PG, et al. Microevolution of antimicrobial resistance and biofilm formation of Salmonella Typhimurium during persistence on pig farms. Sci Rep. 2019;9:8832. Available from: https://doi.org/10.1038/s41598-019-45216-w
- Pande VV, McWhorter AR, Chousalkar KK. Salmonella enterica isolates from layer farm environments are able to form biofilm on eggshell surfaces. Biofouling. 2016;32(7):699-710. Available from: https://doi.org/10.1080/08927014.2016.1191068
- Guéneau V, Plateau-Gonthier J, Arnaud L, Piard JC, Castex M, Briandet R. Positive biofilms to guide surface microbial ecology in livestock buildings. Biofilm. 2022;4:100075. Available from: https://doi.org/10.1016/j.bioflm.2022.100075
- Corrégé I, Hyronimus B, Roulleau X, Hémonic A. Use of a bacterial complex on the surfaces of farrowing and post‐weaning rooms: impact on digestive health and growth performance of piglets. Journées Rech Porcine. 2014;46:181-182.
- IFIP-institut du porc. Mémento de l’éleveur de porc. 7th ed. Salaün Y, coord. IFIP; 2013.
- Hu CH, Gu LY, Luan ZS, Song J, Zhu K. Effects of montmorillonite–zinc oxide hybrid on performance, diarrhea, intestinal permeability and morphology of weanling pigs. Anim Feed Sci Technol. 2012;177:108-115. Available from: https://doi.org/10.1016/j.anifeedsci.2012.07.028
- Sharma S, Mohler J, Mahajan SD, Schwartz SA, Bruggemann L, Aalinkeel R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023;11. Available from: https://doi.org/10.3390/microorganisms11061614
- Joshi RV, Gunawan C, Mann R. We Are One: Multispecies Metabolism of a Biofilm Consortium and Their Treatment Strategies. Front Microbiol. 2021;12. Available from: https://doi.org/10.3389/fmicb.2021.635432
- Guéneau V, Rodiles A, Frayssinet B, Piard JC, Castex M, Plateau-Gonthier J, et al. Positive biofilms to control surface-associated microbial communities in a broiler chicken production system - a field study. Front Microbiol. 2022;13:981747. Available from: https://doi.org/10.3389/fmicb.2022.981747
- Pluske JR, Hampson DJ, Williams IH. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci. 1997;51(1-3):215–236. Available from: https://doi.org/10.1016/S0301-6226(97)00057-2
- Pluske JR, Miller DW, Sterndale SO, Turpin DL. Associations between gastrointestinal-tract function and the stress response after weaning in pigs. Anim Prod Sci. 2019; 59(11):2015-2022. Available from: https://doi.org/10.1071/AN19279
- Li X, Xiong X, Wu X, Liu G, Zhou K, Yin Y. Effects of stocking density on growth performance, blood parameters and immunity of growing pigs. Anim Nutr. 2020;6(4):529-534. Available from:. https://doi.org/10.1016/j.aninu.2020.04.001
- Zhang D, Ji H, Liu H, Wang S, Wang J, Wang Y. Changes in the diversity and composition of gut microbiota of weaned piglets after oral administration of Lactobacillus or an antibiotic. Appl Microbiol Biotechnol. 2016;100:10081-10093. Available from: https://doi.org/10.1007/s00253-016-7845-5.
- Rhouma M, Beaudry F, Thériault W, Letellier A. Colistin in pig production: chemistry, mechanism of antibacterial action, microbial resistance emergence, and one health perspectives. Front Microbiol. 2016;7:1789. Available from: https://doi.org/10.3389/fmicb.2016.01789
- Chee-Sanford JC, Mackie RI, Koike S, Krapac IG, Lin YF, Yannarell AC, et al. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J Environ Qual. 2009;38(3):1086-1108. Available from: https://doi.org/10.2134/jeq2008.0128
- Chen L, Xu Y, Chen X, Fang C, Zhao L, Chen F, et al. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front Microbiol. 2017;8:1688. Available from: https://doi.org/10.3389/fmicb.2017.01688
- Inoue R, Tsukahara T, Nakanishi N, Ushida K. Development of the intestinal microbiota in the piglet. J Gen Appl Microbiol. 2005;51:257–265.
Available from: https://doi.org/10.2323/jgam.51.257 - Massacci FR, Berri M, Lemonnier G, Guettier E, Blanc F, Jardet D, et al. Late weaning is associated with increased microbial diversity and Faecalibacterium prausnitzii abundance in the faecal microbiota of piglets. Anim Microbiome, 2020;2(2).
Available from: https://doi.org/10.1186/s42523-020-0020-4 - Dou S, Gadonna-Widehem P, Rome V, Hamoudi D, Rhazi L, Lakhal L, et al. Characterisation of Early-Life Faecal Microbiota in Susceptible and Healthy Pigs to Post-Weaning Diarrhea. PloS one. 2017;12(1):e0169851. Available from: https://doi.org/10.1371/journal.pone.0169851
- Urubschurov V, Janczyk P, Pieper R, Souffrant W. Biological diversity of yeasts in the gastrointestinal tract of weaned piglets under different farm conditions. FEMS yeast research. 2009;8(8):1349-1356. Available from: https://doi.org/10.1111/j.1567-1364.2008.00444.x
- Konstantinov SR, Awati A, Smidt H, Williams BA, Akkermans AD. Specific response of a novel and abundant Lactobacillus amylovorus-like phylotype to dietary prebiotics in the guts of weaning piglets. Appl Environ Microb. 2004;70(7):3821–3830.
Available from: https://doi.org/10.1128/AEM.70.7.3821-3830.2004 - Martineau GP, Morvan H. Maladies d’élevage des porcs: diagnostics, causes, traitements. 2nd ed. France Agricole Editions. 2010.
- Fairbrother JM, Gyles CL. Colibacillosis, in: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, eds. Diseases of swine. 10th ed. Wiley-Blackwell; 2012:723-747.
- Jenkins TP, Ács N, Arendrup EW, Swift A, Duzs A, Chatzigiannidou I, et al. Protecting the piglet gut microbiota against ETEC-mediated post-weaning diarrhoea using specific binding proteins. npj Biofilms Microbiomes 2024;10(42).
Available from: https://doi.org/10.1038/s41522-024-00514-8
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
