International Journal of Veterinary Science and Research
Minimal Protective Antibody Titers Elicited in Sheep by RVFV MP-12 and arMP-12ΔNSm21/384 Vaccine Candidates
Douglas M Watts1*, Jonna B Westover2, Pedro M Palermo3, Thomas P Monath4, Kevin W Bailey2, George E Bettinger1, Darci R Smith4, John C Morrill5, Phillip R Pittman6, Jeanette Orbegozo1 and Brian B Gowen2
2Institute for Antiviral Research and Department of Animal, Dairy, and Veterinary Sciences, Utah State University, Logan, UT, USA
3Department of Microbiology and Immunology, Naval Medical Research Command, Biological Defense Research Directorate, Fort Detrick, USA
4Crozet BioPharma LLC, Devens, MA, USA
5Department of Microbiology and Immunology, University of Texas Medical Branch at Galveston, Galveston, TX, USA
6Department of Clinical Research, U.S. Army Medical Research Institute of Infectious 27 Diseases (USAMRIID), Fort Detrick, Frederick, USA
Cite this as
Watts DM, Westover JB, Monath TP, Palermo PM, Bailey KW, Bettinger GE, et al. Minimal Protective Antibody Titers Elicited in Sheep by RVFV MP-12 and arMP-12ΔNSm21/384 Vaccine Candidates. Int J Vet Sci Res. 2024;10(3):046-062. Available from: 10.17352/ijvsr.000149Copyright License
© 2024 Watts DM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The live attenuated Rift Valley Fever Virus (RVFV) vaccine candidates, RVFV MP-12, and the recombinant derivative, RVFV arMP-12ΔNSm21/384 (MP-12NSm-del), are among the most promising next-generation domestic ruminant vaccine candidates. While both vaccines consistently elicit protective neutralizing Antibodies (nAb) in domestic ruminants, the minimal protective antibody titer is unknown. Therefore, we conducted studies to determine the minimal protective nAb titers elicited in sheep by these vaccines using a mouse model. The approach involved the transfer of serum samples obtained from sheep vaccinated with the MP-12 and MP-12NSm-del vaccines to 6- to 8-week-old BALB/c mice. The sheep nAb titers ranged from 20 to 640 at the time of transfer. A blood sample was obtained from each mouse 24 hours post-transfer to determine the nAb titer 2 hours before challenging each animal with a lethal dose of virulent RVFV (strain ZH501). All challenged mice were observed daily for 21 days for morbidity and mortality. The lowest nAb titer that protected the animals was interpreted as an estimate of the minimal protective efficacy of the vaccine. The results indicated that nAb titers as low as 10 to 20 elicited by the MP-12 and MP-12NSm-del vaccine candidates in sheep 10 days post-vaccination afforded protection to the mice. However, the nAbs elicited in one sheep by MP-12 before day 10 post-vaccination and ranging in titer from < 5 to 40 only afforded protection to 3 out of 18 mice, and therefore suggested that innate and/or the cellular immune response were also needed for protection during early RVFV infection. The findings further support these RVFV candidate vaccines as potential veterinary vaccines for domestic ruminants and offer a promising BALB/c mouse RVFV challenge model as a surrogate for evaluating the protective nAb response elicited by RVFV vaccines.
Introduction
Rift Valley Fever (RVF) is a multi-species mosquito-borne emerging viral disease caused by Rift Valley fever virus (RVFV), a virion with a small, medium, and large RNA segment of the genus Phlebovirus, family Phenuiviridae, order Bunyavirales [1]. Epizootics and epidemics of RVF occur concurrently at irregular intervals of several years, affecting the health of humans and animals as well as food security, and socio-economic stability in enzootic countries of Africa and the Arabian Peninsula [2,3]. While veterinary vaccines are the most effective control strategy for RVF among domestic ruminants, efforts have had limited success because of safety issues and/or limited protective efficacy of the available licensed vaccines [4-6]. Also, the more common approach has been to delay vaccinating animals until the recognition of the onset of epizootics, an approach that is not recommended because of the risk of spreading RVFV via the use of contaminated needles and the potential for the generating recombinant and reassortant genotypes [7-9].
Several next-generation, live attenuated veterinary vaccine candidates have been developed to prevent RVF among domestic ruminants [5,6,9,10]. Among the live attenuated vaccines, the mutagenized RVFV MP-12 (MP-12) and the recombinant derivative MP-12NSm-del are promising candidates [9]. The MP-12 vaccine was developed by mutagenizing a pathogenic wild-type RVFV strain (ZH548) isolated from a patient in Egypt [11]. The MP-12 vaccine virus strain was mutagenized by serial passages in tissue culture and serial cloning in the presence of the chemical mutagenic agent, 5-fluorouracil. The viral S segment from the 12th serial MP-12 passage acquired one nucleotide change in the intergenic region and three changes in the S segment non-structural (NSs) protein coding region that resulted in a single-amino-acid substitution from valine to alanine at position 513. This point mutation led to reduced virulence and reduction in plaque size of the virus suggesting that this mutation and possibly mutations in other gene segments were responsible for the attenuation [11-15]. As a result of these mutations, especially the point mutation in the S segment, the attenuated MP-12 was found to be a potent IFN-α/β inducer that protected rodents and non-human primates during the early onset of RVFV infection. However, the MP-12 vaccine was not designed to distinguish naturally infected animals from vaccinated animals (DIVA). Therefore, reverse genetics were employed to use the MP-12 parent vaccine virus to develop a recombinant RVFV MP-12NSm-del vaccine candidate with nucleotides 21 - 384 deleted from the M segment non-structural (NSm) protein to serve as a potential DIVA vaccine and to reduce the possibility of genetic reversion [16-18].
Experimental studies showed that the MP-12 vaccine was safe and immunogenic in nonhuman primates, lambs, pregnant ewes, sheep, fetal and neonatal bovids, and human volunteers in the United States [5,9], and safe and immunogenic among sheep, goats, and cattle in Tanzania [19-21]. An exception was the results of a study that showed sheep vaccinated with MP-12 during the early stages of pregnancy aborted and delivered malformed lambs; however, these results have not been confirmed [22]. Also, experimental studies involving sheep and calves, including pregnant sheep in the United States and Canada, demonstrated that the RVFV MP-12NSm-del vaccine was safe and effective [23-25]. The study in pregnant ewes showed that the MP-12NSm-el vaccine elicited a robust sustained neutralizing antibody (nAb) response that was comparable to the parent MP-12 vaccine [24]. Experimental vaccine trials in Tanzania and Morocco showed that the MP-12NSm-del candidate was immunogenic among sheep, goats, and cattle [26-28], but was suspected of causing teratogenicity in pregnant sheep in Morocco [29]. The animals used in the MP-12 and RVFV MP-12NSm-del vaccine trials in Tanzania and Morocco were not challenged with virulent RVFV to assess the efficacy of the vaccines because of the lack of appropriate biosafety containment facilities.
Studies conducted to evaluate the MP-12 and RVFV MP-12NSm-del vaccines in domestic ruminants have involved vaccination trials to determine the nAb response and challenge of vaccinated animals with virulent RVFV to assess protective efficacy. While the challenge of vaccinated animals is the most widely accepted approach for estimating the protective efficacy of RVFV vaccines, such trials, as experienced in our African studies, are not always possible due to the unavailability of large animal-enhanced Biosafety Level 3 containment facilities and the inability to comply with Select Agent regulations required for possessing and working with virulent RVFV [30]. In the absence of such challenge studies, an assessment of the protective efficacy can be made by comparing nAb titers elicited by vaccines to the titers that have been reported to afford protection during small and large animal challenge studies [20,23,28,31-35]. However, estimates based on actual challenge studies involving virulent RVFV have not provided an understanding of the minimal antibody titers required to afford protection by vaccines. Therefore, the minimal nAb titer that affords protection has not been defined and represents a gap in the development and evaluation of veterinary RVFV vaccines. An RVFV vaccine that elicits a very rapid protective immune response at a low level as found early after immunization will be critical, especially during the onset of an outbreak, to protect large animals from infection.
As described in this report, an alternative model was employed to overcome the limitations of using large domestic animals in challenge studies to obtain a better understanding of the extent of protection afforded by neutralizing antibodies to domestic ruminants against RVF disease. The approach used a lethal RVFV murine challenge model for determining the minimal protective antibody titers elicited by MP-12 and RVFV MP-12NSm-del vaccine candidates. Estimates of protective efficacy were determined by correlation of survival rates of the mice that received the RVFV antibody-positive vaccinated sheep serum sample before challenge with virulent RVFV in comparison to survival rates of animals receiving pre-immune antibody-negative serum. This approach using a murine model to determine the protective efficacy of RVFV vaccine candidates could be applicable as a surrogate for evaluating other promising RVFV vaccine candidates in sheep as well as other larger domestic animal species.
Materials and methods
Experimental animals
Female 6- to 8-week-old BALB/c mice (Charles River Laboratories, Wilmington, MA) were acclimated for 7 days and fed Harlan Lab Block and tap water ad libitum before use in the experiments. Balb/c mice were used because this strain of mice infected with RVFV developed both acute-onset hepatitis and delayed-onset encephalitis disease that closely resembles the more severe form of RVF in humans and livestock, and therefore represents a good small animal model for the evaluation of potential therapeutics and vaccines for RVFV [36]. The animal procedures involved in this study complied with USDA guidelines and were conducted at the AAALAC-accredited laboratory animal research facilities at Utah State University under protocol #10248 which was approved on October 15, 2019, by the Utah State University Institutional Animal Care and Use Committee.
Viruses
The molecular clone of a virulent strain of RVFV, strain ZH501, was obtained from Dr. Stuart Nichol (CDC, Atlanta, GA). The clone was used to prepare a stock virus with an infectivity titer of 1.1 × 108 Plaque-Forming Units (PFU) per mL. By one passage in BSR-T7/5 cells and 3 passages in Vero E6 cells. The BSR-T7/5 cells baby hamster kidney- cells that express T7 RNA polymerase and were kindly provided by Dr. K. Conzelman (Max-von Pettenkofer-Institute, Munchen, Germany). The RVFV virus stock was diluted in sterile Minimum Essential Medium (MEM) and 0.1 ml containing ~100 PFU/mL was *inoculated by the Subcutaneous (SC) route into each mouse on the ventral, right side of the abdomen.
The MP-12 vaccine was derived by mutagenizing a virulent wild-type RVFV strain (ZH548) isolated from a patient in Egypt [17]. However, the MP-12 vaccine was not formulated with biomarkers required to distinguish naturally infected animals from vaccinated animals (DIVA). Therefore, the MP-12 parent vaccine virus was used to develop a recombinant RVFV MP-12NSmdel vaccine with a biomarker that included a deletion at nucleotides 21 - 384 in the NSm gene to serve as a potential DIVA vaccine [16-18].
Serum samples
Sheep serum samples were obtained during a study conducted in 2011 from F1 Suffolk-Rambouillet crossbred pregnant ewes (30-50 days of gestation) that had been sham-vaccinated with MEM or with a single SC injection of 1 x 105 - PFU of the MP-12 vaccine or the RVFV MP-12NSm-del vaccine during an experiment to determine the safety and immunogenicity of these vaccines in pregnant sheep [23]. The serum samples were collected 7 days pre-vaccination, the day of but before vaccination, and at various intervals from days 1 through 69 Post-Vaccination (PV) of sheep and stored at -80 °C until selected samples were used in this study. The samples selected for use were obtained on days 0, 6, 8, 10, 11, and 49 PV from sheep vaccinated with MP-12 and had reciprocal plaque reduction neutralization antibody (PRNT80) titers of 20, 40, 80, 160, 320 and 640. The samples selected for use from animals vaccinated with RVFV MP-12NSm-del vaccine were obtained on days 0, 12, 21, and 28 and had reciprocal PRNT80 nAb titers of 20, 40, 80 and 160. The donor sheep that provided the serum samples is included in the methods for experiments 1, 2, and 3, below. The nAb-negative serum samples were obtained from sheep on day 0 before vaccination and were pooled and used as an RVFV nAb-negative control. While the nAb PRNT80 titers were determined in 2011-12 during the vaccine trial [23] that generated the serum samples used in this study, the samples were retested to confirm the nAb PRNT80 titers before use in this study by testing two-fold dilutions of the samples using the PRNT80 procedures described below.
Plaque reduction neutralization test
The Plaque Reduction Neutralization Test (PRNT80) was used to determine the antibody titers of the serum samples obtained from the sheep vaccinated with the MP-12 and RVFV MP-12NSm-del vaccines. The procedure was performed by initial heat inactivating the serum samples at 56 °C for 30 min and tested for nAb to the MP-12 virus in Vero E6 cells as described previously [20]. Briefly, equal volumes of 2-fold dilutions ranging from 1:5 through 1:1280 of each sample were prepared in MEM and incubated overnight at 4 °C with an equal volume of MEM containing 75 PFUs of the MP-12 virus. On the following day, 50 µL of the virus/serum dilution mixture was inoculated in duplicate onto Vero E6 cells grown in 24-well plates. Cultures and inocula were incubated for 1 h at 37 °C and 5% CO2. A mixture of 1% SeaKem agarose (VWR, Radnor, PA) with an equal volume of 2X Eagle’s Basal Medium with Earle’s salts (EBME), (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES), sodium bicarbonate, 8% FBS and 1% penicillin/ streptomycin and L-glutamine (Invitrogen, Carlsbad, CA) was then prepared, and 0.5 mL was overlaid onto each cell culture. The cultures were incubated for 3 days at 37 °C and 5% CO2 and then stained with a 0.33% neutral red solution and incubated for 4-6 h to identify plaques. The highest dilution of serum that reduced the number of plaques relative to the negative sheep serum control by 80% was considered to be the PRNT80 nAb titer.
Experimental design and methods
Experiment 1: The serum samples with known RVFV nAbs used in this pilot experiment were obtained from sheep that were previously vaccinated, as described above, with the MP-12 and RVFV MP-12NSmdel vaccine candidates [23]. The reason for selecting the antibody-positive sheep serum samples with different titers, ranging from the lowest to the highest titers (20 to 640) was to take advantage of the availability of archived sera with a broad range of antibody titers. Sheep sera with different antibody titers were transferred to 6- to 8-week-old BALB/c mice which were then challenged with a lethal dose of virulent RVFV to determine the lowest antibody titer that afforded protection. In the initial pilot experiment, the sheep serum samples available included those that had been obtained from sheep #25 on Post-Vaccination (PV) days 6, 8, and 49 with reciprocal nAb titers of 20, 160, and 640, and a sample from sheep #26 on PV day 11 with a titer of 320 and samples from sheep #28 on PV days 10 and 11 with titers of 40 and 80, and a pool of antibody-negative samples for use as controls. Mice were weighed one day before the passive transfer of these sheep sera and grouped so that the average weight per the experimental group of 4 mice each across the entire experiment varied by less than 1.5 g. Each of the 6 serum samples of known RVFV antibody titer was thawed and 0.2 mL volumes of each sample were passively transferred via Intraperitoneal (IP) injection. A similar volume of pooled RVFV antibody-negative sheep serum was passively transferred to a group of 4 mice to demonstrate that the challenge RVFV dose resulted in lethal disease and 2 animals were sham-infected with MEM to serve as controls. Whole blood (150 µl) was obtained from the submandibular vein of the mice at 24 h post-transfer of antibody and centrifuged to obtain serum for estimating the nAb titer by PRNT in Vero cells. At 2 h after obtaining blood samples, i.e. at 26 h post-transfer of antibody, all mice were transferred to a BSL-3+ Select Agent containment laboratory and challenged with 100 PFU of RVFV (ZH501) by SC injection, the dose at which approximately 90% of the challenged mice succumb (Lethal Dose90 (LD90)) based on titration of the virus stock in BALB/c mice). The challenged mice were observed once daily for 21 days for clinical signs and/or terminal signs of mortality. The indicators of the protection of mice were the absence of any signs of illness, such as weight loss, ruffled fur, hunched back, immobility, or the inability to consume food and water. The lowest nAb titer that protected the animals was interpreted as an estimate of the minimal protective efficacy of the vaccine.
Experiment 2: Our findings for the above pilot experiment indicated that the RVFV MP-12 virus antibody elicited in sheep by the MP-12 vaccine afforded protection when passively transferred to mice and challenged with a lethal dose of virulent RVFV. After the transfer of antibodies with different titers to mice, a titer in the mice of 20 or greater afforded protection to 75% (3/4) or more of the mice against challenges with the RVFV ZH501 virus. Based on this observation, a second experiment was performed to confirm the minimal antibody titer required to afford protection to the mice. The methods were the same as for the first experiment except that the MP-12 antibody-positive serum samples elicited in sheep had titers of 40, 160, or 640. These samples included those that had been obtained from sheep #25 on PV day, 8 with reciprocal nAb titer of 160 a sample from the same sheep on PV day 49 with a titer of 640, and a sample from sheep #28 on PV day 10 with a titer of 40, and a pool of antibody-negative samples for use as controls. Each sample was transferred to groups of 10 mice for a challenge with RVFV ZH501. In addition, pooled samples of antibody-negative serum samples were transferred to a group of 10 mice to demonstrate that the RVFV challenge dose caused a lethal infection in these negative control mice. Four sham-infected mice inoculated with MEM vehicles served as uninfected controls. The starting weights of the mice across the experimental groups varied by less than 1.3 g and equal numbers of male and female animals were included per group.
Experiment 3: In this experiment, we utilized the same RVFV mouse infection model as described in experiments 1 and 2 to compare the protection of passively transferred, antibody-positive serum obtained from sheep following vaccination with the RVFV MP-12 and arMP-12ΔNSm21/384 vaccines against RVFV ZH-501 challenge. The methods were the same as for the first and second experiments except that the serum samples selected included MP-12 antibody-positive sheep serum samples with titers of 20, 40, 80, or 160. These samples included those that had been obtained from sheep #27 on PV day 11 with reciprocal nAb titer of 20 a sample from sheep #26 on PV day 10 with a titer of 40, a sample from sheep #28 on PV day 11 with a titer of 80, and as sample from sheep # 27 on PV day 11 with a titer of 160, and a pool of antibody-negative samples for use as controls. Serum samples were passively transferred to groups of 8 or 9 mice, and pooled antibody-negative control serum samples were transferred to 9 mice to demonstrate the lethality of the RVFV ZH501 challenge dose. Four mice were sham-infected with MEM vehicles as additional controls. The starting weights of the mice across the experimental groups varied by less than 1.3 g and equal numbers of male and female animals were included per group.
The arMP-12ΔNSm21/384 serum samples included antibody -positive sheep samples with titers of 20, 40, 80, and 160. These samples included those that had been obtained from sheep #8 on PV day 28 with a reciprocal nAb titer of 20 a sample from sheep #6 on PV day 21 with a titer of 40, a sample from sheep #5 on PV day 12 with a titers of 80, and a sample from sheep # 8 on PV day 28 with a titer of 160, and a pool of antibody-negative samples for use as controls. Serum samples were passively transferred to groups of 8 or 9 mice, and pooled antibody-negative control serum samples were transferred to 9 mice to demonstrate the lethality of the RVFV ZH501 challenge dose. Four mice were sham-infected with MEM vehicles as additional controls. The starting weights of the mice across the experimental groups varied by less than 1.5 g and equal numbers of male and female animals were included per group.
Experiment 4a: Enzyme-Linked Immunosorbent IgM and IgG Assay (ELISA): Sheep serum samples were tested for RVFV IgM and IgG antibodies by using a commercial ELISA kit ID Screen® Rift Valley Fever Competition Multi-species diagnostic test (IDvet, Innovative Diagnostics, Grabels, France). The sera samples were diluted 1:2 in MEM and each dilution was added to each well of 96-well plates coated with a recombinant RVFV nucleoprotein (NP) and incubated for 1 h at 37 °C. After washing the wells, 100 µL of an anti-NP peroxidase conjugate was added to detect NP bound by the test serum. The reaction was allowed to incubate for 30 min at room temperature and then, 100 µl of 3,3’,5,5’-Tetramethylbenzidine (TMB) substrate solution was added to each well. After 15 minutes, 100 µl of 0.16 M sulfuric acid was added to each well to stop the reaction, and the absorbance was read at an Optical Density (OD) of 450 nm. OD values from the duplicate samples were subtracted to obtain a net OD and the percentage of the ratio of sample OD and positive control OD (S/P%) were calculated. The samples with an S/P% ≤ 40% were considered negative, samples with an S/P% between 40% and 50% were considered doubtful, and samples with an S/P% ≥50% were considered positive for antibodies.
Experiment 4b: Enzyme-Linked Immunosorbent IgM Antibody Capture Assay (ELISA): Sheep serum samples were tested for only RVFV IgM to the NP by using the ID Screen RVFV IgM Capture kit (IDvet, Innovative Diagnostics, Grabels, France) according to the manufacturer’s instructions. The sera samples were diluted 1:5 in MEM and dispensed in duplicates into each well of a 96-well microplate pre-coated with anti-bovine-ovine-caprine IgM polyclonal antibody and incubated at 37 °C for 1 h. After the incubation period, the microplate wells were washed with 50 μL of RVFV nucleoprotein and/or diluent buffer and then incubated at 37 °C for 1 h. Then plates were washed again and 50 μl of anti-RVFV NP horseradish peroxidase (HRP) conjugated antibody solution was added to each well and incubated at 37 °C. Again, the wells were washed and 100 μL of the 3,3’,5,5’-tetramethylbenzidine (TMB) substrate solution was added to each well and then incubated for 15 min at room temperature. Finally, 100 μl of 0.16 M sulfuric acid was added to stop the reaction and the absorbance was read at 450nm. OD values from the duplicate samples were subtracted to obtain a net OD and the percentage of the ratio of sample OD and positive control OD (S/P%) were calculated. The samples with an S/P% ≤ 40% were considered negative, samples with an S/P% between 40% and 50% were considered doubtful, and samples with a S/P% ≥50% were considered positive for antibodies.
Statistical analysis
The two-tailed Fisher exact test was used for the analysis to determine statistically significant differences, if any, between the survival of mice that received serum samples from RVFV vaccinated sheep versus mice that received serum samples from normal unvaccinated sheep following challenge with a lethal dose of virulent RVFV. The Mantel-Cox log-rank test was used for the analysis of Kaplan-Meier survival curves using Prism 10 (GraphPad Software, La Jolla, CA). The duration of survival of mice that received MP-12 antibody was compared statistically with the duration of survival of the mice that received normal sheep serum.
Results
Experiment 1: This pilot experiment was performed to determine the lowest concentration of RVFV MP-12 nAb in serum samples obtained from MP-12 vaccinated sheep that afforded protection after passive transfer to BALB/c mice challenged with a lethal dose of virulent RVFV. The MP-12 antibody titer in the sheep serum samples before passively transferred to each mouse, the antibody titers detected in the serum samples obtained from mice 24 h after passive transfer, and the survival rate of mice following challenge with virulent RVFV are presented as supplementary data in Table S1. All 4 of the mice treated with antibody-negative serum #26D0 (obtained from sheep #26 on Day 0 PV) 1 day before the virulent RVFV challenge succumbed to virus infection by day 10 post-infection (PI) (Table S1). There was a correlation between the titer of the MP-12 antibody in the original sheep serum samples, the antibody titer measured in the sera of recipient mice 24 h after transfer, and the RVFV infection survival rates (Table S1). A summary of the antibody protection data for the sheep sample nAb with reciprocal titers of 20, 40, 80, 160, 320, and 640 is presented in Table 1. The results for the transfer of sheep serum samples #25D6 (sheep #25 Day 6 PV) to 4 mice with a titer of 20 indicated that the titers on transfer to mice decreased to < 5 for 3 mice and to a titer of 5 for one animal, and only one of these animals (< 5) survived the challenge. Upon transfer of antibody with a titer of 40 in sheep serum sample #28D10 to 4 mice, the titer decreased to 20 for each of the 4 mice and 3 of these 4 animals were protected against challenge. Transfer of sheep serum sample #28D11 with a titer of 80 to 4 mice resulted in a titer decrease to 20 for one animal and to 40 for 3 animals, which protected all 4 animals from the lethal RVFV challenge. On transfer of the serum sample #25D8 with a 160 titer to 4 mice, the titer decreased to 5 for one animal, 20 for one animal, and 40 for 2 animals. Of these animals, the 2 with titers of 40 were protected, but the ones with a 20 and 5 titer were not protected against the RVFV challenge. Transfer of the sheep serum samples #26D11 and #25D49 to 4 mice with antibody titers of 320 and 640 respectively led to a decrease in the titers of each group of 4 mice to 40 and 160, and all these animals were protected from challenge with a lethal dose of virulent RVFV.
The survival curve for each group of mice treated in experiment #1 with each of the different concentrations of MP-12 sheep antibody is presented in Figure 1A. Animal weights were also measured during the study. The percent weight change in mice relative to their starting weights on day -1, the day they received the antibody-positive sheep sera, are shown in Figure 1B and are consistent with the survival data. The mice that received the antibody-negative (titer < 10) serum abruptly lost weight after the RVFV challenge as they approached the terminal stages of the disease with the last animal succumbing to the virus infection on day 10 PI. The animals that received sheep serum samples with antibody titers of 20, or the lowest concentrations of antibody tested, also displayed weight loss as they approached the terminal stages of the disease.
Except for the 5 unprotected mice which included 2 mice with a titer of < 5, 2 mice with a titer of 5, and 1 mouse with a titer of 20, all of the other mice with a titer > 20 were protected resulting in a survival rate of 75% (18/24) following challenge with a lethal dose of RVFV. The data obtained from this first experiment were used for the selection of MP-12 antibody-positive sheep serum samples for use in the second experiment to better define the lowest nAb titer that protected mice following the challenge with RVFV.
Experiment 2: The second experiment was performed to confirm results obtained during the first experiment that showed an antibody titer of 20 elicited by MP-12 vaccination of sheep was the lowest titer that afforded protection when immune serum was transferred to mice, except one mouse that was protected with a < 5 titer. A summary of this second experiment, including the nAb titer of the sheep serum that was administered to each mouse, the titers of the MP-12 nAb detected in the mice 24 h after passive transfer, and the survival rate of mice following challenge with a lethal challenge of RVFV is presented as supplementary data in Table S2. All the mice treated with the antibody-negative sheep serum #27D0 and challenged with lethal RVFV succumbed to the virus infection by day 13 PI (Table S2). There was strong protection with passive transfer of the sample with an MP-12 nAb titer of 40. In contrast, the sample with the sheep nAb titer of 160 failed to protect mice against RVFV infection and the titers in mouse serum 24 h post-transfer revealed low nAb titers ranging from < 5 to 10 (Table S2). A summary of the antibody protection data for the nAb titers of 40, 160, and 640 is presented in Table 2. The results for sheep serum sample #28D10 with a titer of 40 indicated that on transfer to 10 mice, the titer decreased to 10 for 2 mice and 20 for 8 mice. All these animals except one with a titer of 10 were protected against a lethal challenge dose of virulent RVFV. On transfer of the serum sample #25D8 with a 160 titer to 10 mice, the titers decreased to < 5 for one mouse, a titer of 5 for 5 mice, and a titer of 10 for 4 mice. None of these animals survived the challenge. This serum sample (#25D8) with a 160 nAb titer was the same as used in the first experiment and found to afford protection to 2 of 4 mice and, as stated above for the results of experiment #1, the sample obtained from the same sheep #25 on day 6 only protected 1 of 4 mice. Among the 10 mice that received serum sample #25D49 with a titer of 640, the titer decreased to 80 for 8 mice and 160 for 2 mice. All of these mice survived the lethal RVFV challenge. Overall, the antibody protection rate was 63% (19/30) and this lower rate can be attributed primarily to the failure of sera samples from sheep #25 that were obtained before day 10 PV to afford protection to the animals.
The survival curve for each group of mice treated with the different MP-12 nAb sheep sera is presented in Figure 2A. Animal weights were measured daily during the experiment and the percent weight change in mice relative to their starting weights on day-1, the day they received the sheep immune serum, is presented in Figure 2B and is consistent with the survival data. The mice that received the non-immune serum and the 10 mice that received sheep serum #25D8 with a titer of 160 but decreased on transfer to mice to titers ranging from < 5 to 10, abruptly lost weight as they approached the terminal stages of disease. Except for sheep serum #25D8, the mice that received sheep serum with MP-12 nAb titers of 40 or 640 generally gained weight at a trajectory similar to that of the sham-infected normal control mice.
This second experiment provided strong evidence that passively transferred MP-12 nAb from sheep serum to BALB/c mice significantly protected against challenges with a lethal dose of RVFV. The minimal antibody titers that protected mice against the challenge ranged from 10 to 20 for 8 of 10 mice that survived the lethal RVFV challenge after passive transfer of sheep serum with a titer of 40. The overall lower protection rate of 63% (19/30) was attributed to the failure of the serum sample taken on day 8 PV from sheep #25 with a titer of 160 that decreased on transfer to 10 mice to a titer of 10 to undetectable (< 5). The serum sample #25D8 was repeated in experiment #2 because in experiment #1, on transfer of the samples to 4 mice, the titer decreased to 40 for 2 mice, 20 for 1 mouse, and 5 for 1 mouse, and only protected the mice with a titer of 40, but not the mice with a titer of 5 and 20. Overall, samples from #25D6 and #25D8 failed to protect 12 of 14 mice, thus suggesting the possibility of the loss of titer due to repeated thawing of the sample or that antibodies elicited in sheep before day 9 were not protective.
Experiment #3: This experiment was performed to compare the ability of passively transferred nAbs elicited in sheep with MP-12 or MP-12NSmdel vaccinations to protect against RVFV ZH501 infection. A summary of this experiment, including the nAb titer of the sheep serum that was administered to each mouse, the titers of the MP-12 and MP-12NS-del nAb detected in the mice 24 h after passive transfer, and the survival rate of mice following challenge with a lethal dose of RVFV are presented in Table S3 and Table S4, respectively. All but one of the 9 mice treated with the RVFV antibody-negative serum samples succumbed to the RVFV challenge by day 10 PI (89% mortality). The correlation between nAb detected in the mouse serum after passive transfer of anti-MP-12 nAb titers of 20, 40, 80, and 160 and protection was not as clear (Table S3). A summary of the antibody protection data for MP-12 sheep sera samples with nAb titers of 20, 40, 80, and 160 is presented in Table 3. After the transfer of the sera sample #27D11 with a titer of 20 to 8 mice, the titer decreased to < 5 for all mice, and 6 of 8 mice were protected against a lethal challenge of RVFV. Among 9 mice that received serum sample #26D10 with a titer of 40, the titer decreased to < 5 for 6 mice, 5 for 2 mice, and 10 for 1 mouse. Six of 9 of these mice survived the challenge. Of the 9 mice that received sheep serum sample #28D11 with a titer of 80, the titer decreased to 10 for 3 mice, 20 for 5 mice, and 40 for 1 mouse. Eight of 9 of these mice survived the challenge. The titer in 8 mice that received serum sample #27D11 with a titer of 160 decreased to a titer of 5 for 1 mouse and to a titer of 10 for 7 mice, and 7 of 8 of these mice survived the challenge. This sheep serum sample #27D11 with a titer of 160 was collected on day 11 following the vaccination of sheep with the MP-12 vaccine. In contrast, the sheep serum sample #25D8 with a 160 nAb titer used in experiments 1 and 2 was obtained from vaccinated sheep on day 8 PV and failed to protect 12 of 14 mice, further suggesting the possibility that antibody elicited in sheep before day 10 was not protective in this study.
The data presented in Figure 3A shows the survival curves of the mice treated with serum from sheep immunized with MP-12 and Figure 3B shows the corresponding percent weight change. Notably, for the 40 and 160 nAb titer sheep serum, there was a trend towards lower titers of nAb in mice 24 h after passive transfer with the MP-12 samples as compared to the nAb titers elicited by the MP-12NSm-del vaccine (Figure 4).
A correlation was observed between the MP-12NSm-del sheep serum nAb titers of 20, 40, 80, and 160 and the titers detected in the serum samples of recipient mice and survival outcomes (Table S4, Table 4). A summary of the protection data for the nAb titers of 20, 40, 80, and 160 is presented in Table 4. Among the mice treated with these nAb-positive serum samples elicited in sheep by the MP-12NSm-del vaccine, the titers after transfer to mice ranged from < 5 to 40 (Table 4). After the transfer of the sheep serum sample #8 Day 28 (#8D28) with a titer of 20 to 8 mice, the titer decreased to < 5 for 4 mice and to a titer of 5 for 4 mice. However, 3 of these mice with a titer of 5 were protected against the lethal RVFV challenge. Among the 9 mice that received serum samples #6D21 with a titer of 40, the titer decreased to 5 for 1 mouse and to 10 for 8 mice. All nine of these mice survived the RVFV challenge. After transfer of the serum sample #5D12 with a titer of 80 to 9 mice, the titer decreased to 10 for 4 mice and to a titer of 20 for 5 mice, and all but one of the 9 mice with a titer of 20 were protected against the lethal challenge dose of RVFV. Among the 9 mice that received sera samples #8D28 with a titer of 160, the titer decreased to < 5 for 1 mouse, to 10 for 1 mouse, 20 for 6 mice, and 40 for 1 mouse. All but one of the mice with a titer of < 5 survived the challenge. Overall, the serum samples tested were obtained from sheep between post-vaccination days 12 and 28 (Table 4). None of the mice with a titer of < 5 survived, but protection was afforded to 80% (4/5) of mice with a titer of 5. Of the remaining serum samples, 100% (10/10) of mice with a titer of 10, 91% (10/11) with a titer of 20, and 100% (1/1) with a titer of 40 were protected from challenge. This third experiment demonstrated that the minimal titer of MP-12 antibody that protected mice from challenge with a lethal dose of RVFV varied from < 5 to 20 and the minimal titer of MP-12NSm-del antibody ranged from 5 to 10. Although nAb-positive serum samples obtained from sheep vaccinated with both the MP-12 and MP-12NSm-del vaccines with a titer as low as < 5 and 5, respectively, protected mice from challenge with a lethal dose of virulent RVFV, the overall minimal nAb protection titer ranged from 10 to 20 for serum samples obtained from sheep vaccinated with both vaccine candidates. Overall, 80% (28/35) of the serum samples with a titer ranging from < 5 – 40 afforded protection to mice.
The survival curve for each group of mice treated with each of the different concentrations of sheep serum with MP-12NSm-del nAb and challenged with a lethal dose of virulent RVFV is presented in Figure 5A, and Figure 5B shows the corresponding percent weight change. Overall, the protection afforded by the MP-12 and MP-12 MP-12NSm-del nAbs against RVFV infection was comparable across the different levels of nAb titers present after passive transfer with no statistically significant differences observed by log-rank analysis (Figure 6).
As described above and summarized in Table 5, only 17% (3/18) of mice passively transferred with serum from sheep #25 collected on days 6 and 8 PV were protected from challenge with virulent RVFV. In contrast, 76% (61/80) of mice receiving serum samples from sheep collected on days 10, 11, and 49 PV survived RVFV infection (Table 5). The overall protection rate afforded by all 88 serum samples was 74% (65/88). While the nAb positive sera samples obtained from sheep vaccinated with the MP-12 and with the MP-12NSm-del vaccine candidates with a titer as low as < 5 and 5, respectively, protected mice, the overall minimal nAb protective titers ranged from 10 to 20 for both vaccine candidates.
Experiment 4: As an effort to understand the poor protection rate afforded to mice by the serum samples obtained from MP-12 vaccinated sheep #25 before day 10 PV, all serum samples obtained from sheep #25, #26, # 27 and #28 used in this study were tested for IgM antibody and total IgM and IgG antibodies to the RVFV nucleoprotein (NP) and the nAb data for these samples were obtained from previously published data obtained in a study that tested sera samples from vaccinated sheep at intervals through 56 days PV (34). As shown in Table 6, the results for serum samples obtained from sheep # 25 on days 6 and 8 PV were negative for IgM antibody to the NP. In contrast, the IgG antibody to the NP was first detected on day 10 PV and nAb was first detected on day 5 PV and both the IgG and nAb remained detectable throughout the duration or day 56 PV of the study. In contrast to the results for testing serum samples from animal #25, serum samples from the other animals (#26, 27, 28), which included all samples used in this study that were taken on day 10 and after vaccination, were positive for IgM antibody to NP as early as day 7 or 8 PV and remained positive until days 12, 14 and 28 PV. Whereas the IgG antibody to NP was first detected on day 7 PV and nAb on day 6 and 7 PV, and both the IgG antibody and nAb remained positive through day 56 PV. Thus, the results for serum samples from sheep #25 that failed to protect the animals were negative for NP IgM antibody and the NP IgG antibody was not detectable until day 10 PV, or for 2 to 3 days after the detection of NP IgG antibody in sera of the other 3 animals. Also, nAb was detected in serum samples from all animals including the detection antibody on days 5 for #25, 7 for #26, 6 for #27, and 7 for #28, or in 2 animals before the NP IgG antibody was detected. Thus, the difference in the immune response based on testing serum samples obtained on days 6 and 8 PV from the #25 animal in comparison to testing results for animals #26, 27, and 28 was that the #25 samples were negative for IgM antibody throughout the 56-day study period and were negative for IgG antibody to NP until day 10 PV.
Discussion
Several next-generation RVFV veterinary vaccines are under development for the prevention of RVF among domestic ruminants [5,6,9,10]. Estimates of the protective efficacy of some of these candidate vaccines have been determined by vaccinating and subsequent challenge of laboratory animals (rodents and non-human primates) and domestic ruminants with virulent RVFV [33-35]. However, the challenge of animals using virulent RVFV is limited due to the unavailability of appropriate biosafety containment laboratories [19-21,26-29]. As an alternative approach reported herein, a lethal virulent murine challenge model was used in this study to estimate the minimal protective antibody titers elicited in sheep following vaccination with the RVFV MP-12 vaccine. The design involved the selection of RVFV antibody-positive sheep sera samples with different nAb titers elicited on different PV days in sheep with the RVFV MP-12 and MP-12NSm-del vaccine candidates. The passive transfer of these samples to Balb/c mice that were subsequently challenged with a lethal dose of RVFV indicated that the minimal protective antibody titer for both vaccines ranged from 10 to 20. These titers were similar to minimal protective RVFV antibody titers of 10 to 20 elicited in human volunteers by the MP-12 vaccine and in hamsters by an inactivated RVFV NDBR-103 vaccine [37,38]. In the same study involving the NDBR-103 vaccine, only 2 of 10 vaccinated hamsters developed detectable antibodies, but all animals survived the challenge with a lethal dose of virulent RVFV indicating that animals with a titer of < 10 were protected [38]. Our findings also indicated that some animals were protected with serum samples that had values of < 5 antibody titers. The only relevant studies involving the recombinant MP-12NSm-del vaccine indicated that an antibody titer in sheep at the time of challenge of about 100 afforded protection against challenge with a virulent strain of RVFV [25].
As observed for the protective role of nAb elicited in sheep by the RVFV MP-12 vaccine in this study, our findings indicated that the passive transfer of RVFV antibody to mice and challenged with virulent RVFV was a reliable method for evaluating the protective efficacy of nAb against RVF disease. As a result, this method could have advantages over the classical methods of directly challenging large animals with virulent RVFV for evaluating the efficacy of candidate RVFV vaccines. However, very few studies have been designed appropriately to determine the antibody titers at the time of challenge. Also, numerous studies have evaluated the safety and immunogenicity of vaccine candidates, but the protective efficacy could not be evaluated by challenge with virulent RVFV. For example, our studies involving the evaluation of both the MP-12 and the MP-12NSm-del vaccine in African domestic ruminants demonstrated that these candidates elicited nAb [19-21,26-29]. However, estimates of the protective efficacy of the antibody-based on the challenge with virulent RVFV were not possible due to the unavailability of large animal-enhanced Biosafety Level 3 containment facilities and the inability to comply with Select Agent regulations [30]. While the protective efficacy of the antibody titers was unknown, the immune response of the African ruminants to both vaccines was comparable to or higher than the 10 to 20 titers of sheep serum that afforded protection to mice. As such, our findings in this study support the validity of the use of passively transferred RVFV antibodies from vaccinated animals to a mouse model as a reliable and promising approach for use as a surrogate to provide a more cost-effective and concise estimate of protective antibody titer than the classical approach of using small and/or large domestic ruminant RVFV challenge models.
Among the MP-12 antibody-positive sheep serum samples passively transferred to mice, 23 of 88 mice succumbed to challenge infection with a lethal dose of virulent RVFV for an overall protection rate of 74% (65/88). The mice that were not protected included 83% (15/18) that received nAb-positive serum samples obtained from one MP-12 vaccinated sheep (#25) on days 6 and 8 PV, or before day 10 PV. The antibody titers in 15 of the un-protected mice ranged from < 5 to 20 and of the 3 mice that were protected, one mouse had a titer of < 5, and 2 mice had titers of 40. The remaining 8 of the 23 unprotected mice had received antibody-positive serum samples obtained from sheep on days 10 and 11 PV with titers ranging from 5 to 20. Overall, 21 of the 23 mice that were not protected involved animals that had titers ranging from < 5 to 10, including 7 with a titer of < 5, 8 with at titer of 5, and 6 with a titer of 10. An additional 2 unprotected mice had a titer of 20 and all these serum samples were obtained from sheep on days 6 to 11 PV with MP-12. The possibility cannot be excluded that the 26% (23/88) of the passively transferred RVFV antibody-positive sheep serum samples did not protect mice because the transfer of antibody to mice resulted in dilutions as much as < 5 to 160 of the original antibody titers detected in sheep on day 6 to 11 PV of sheep with the MP-12 vaccine. However, dilutions that resulted in < 5 antibody titers were protective on days 10 and 11 PV, suggesting that the lack of protection was due to other reasons.
The failure of RVFV MP-12 elicited antibody in sheep vaccinated on or before day 10 PV to afford protection on passive transfer to 15 of 18 mice was associated with 2 serum samples obtained from sheep #25, one sample on day 6 (#25D6), and one on day 8 (#25D8) PV, and were the only samples tested before day 10 PV in this study. The failure did not appear to be related to the lower nAb titers elicited in sheep early after vaccination with the MP-12 vaccine. For example, only one of 10 samples obtained from sheep before day 10 PV with titers ranging from < 5 to 5 in recipient mice afforded protection to challenged mice, whereas 11 of 16 samples with similar titers obtained from sheep on days 10 and 11 PV protected mice. While the 2 samples from sheep #25 were positive for nAb with titers of 80 (#25D6) and 160 (#25D8) before transfer to mice and ranged from < 5 to 40 after transfer to mice, the failure to afford protection could have been due to the predominance of IgM antibody to Gc/Gn envelope glycoproteins that are known to be elicited earlier than the more potent IgG nAb. Also, since the IgM antibody is much larger than the IgG antibody, this antibody could have either been cleared before mice were challenged or the antibody was poorly neutralizing. In support of this possibility are the results for serum sample #27 which was taken from a sheep on day 11 PV with a titer of 160 before transfer to 8 mice. On the challenge of the mice that had titers ranging from 5 to 10, 7 of the 8 mice survived the challenge suggesting that both IgM and/or IgG antibodies afforded protection to mice. That IgM antibody was predominate before IgG nAb during early RVFV infection is supported by the results of studies that showed IgM antibody to be detectable in sheep infected with virulent RVFV or vaccinated the Smithburn vaccine or non-human primates with MP-12 by day 4 PI and peaked on day 10 in contrast to IgG antibody that was usually detectable a few days later but did not reach peak titers until day 20 post-inoculation [39].
While our observations further demonstrated the critical role of RVFV MP-12 vaccine-elicited nAb for protecting against RVF disease, the observation for sheep #25 suggested that the humoral response of nAb alone was not protective on days 6 and 8 PV, or that higher tittered antibody was required based on the titer of 40 that afforded protection to mice. The higher titer of 40 as opposed to the unprotected antibody titers that ranged from < 5 to 10 for the other 15 unprotected mice suggested that the avidity of the higher concentration of antibody may have been stronger and therefore neutralized virus infectivity. Although antibodies alone failed to afford protection, evidence accumulated indicated that both the cellular and/or innate immune response may be required to afford protection during the early course of infection. As an effort to understand the possible protective role of the early humoral immune response, the serum samples from sheep #25 obtained on days 6 and 8 PV as well as the samples obtained from sheep #26, 27, and 28 were tested for IgM and IgG antibody to the RVFV Np [40]. The results revealed that the sera samples from sheep #26, 27, and 28 were positive for IgM and IgG antibodies to the RVFV NP antigen, but the serum samples from #25 sheep were negative for IgM antibody and the IgG antibody response was delayed, suggesting that the MP-12 vaccine failed to elicit an NP response in this animal. Although nAb to Gc/Gn envelope glycoproteins were detected in sheep #25 on day 5 PV and every day thereafter during the 56-day study period, this antibody as mentioned was not protective on days 6 and 8 PV, but afforded protection on day 49 PV or the only other day that the sera from this sheep was evaluated. In contrast to sheep #25, sera obtained from the other 3 sheep were positive for IgM and IgG antibody to the NP as early as days 7 and 8 PV, but the first sera samples from sheep #26, 27, and 28 that were evaluated for protection were those obtained on day 10 and 11 PV when 88% (38/43) of the samples from these animals afforded protection to mice. Since data are not available for direct comparison of protectiveness, and only involved one animal, the observation must be interpreted with caution and warrant further investigation regarding a possible protective role of nAb early during RVFV infection. However, numerous studies have demonstrated that the immune response to the RVFV immunodominant NP, presumably mediated by cellular immunity afforded partial protection to mice in the absence of nAb antibody [41-44]. The protectiveness during the early course of infection was demonstrated by a study that showed a combination of RVFV non-neutralizing and monoclonal antibodies Gn32 afforded complete protection when transferred to mice 30 minutes after a lethal challenge with virulent RVFV [45]. Also, Fc-mediated effector functions may contribute to the overall antiviral activity which is something our study did not address.
Although the design of this study precluded consideration of the well-documented protective role of the RVFV-mediated innate immune response, the observation suggesting that antibody elicited in sheep by the MP-12 vaccine before day 10 PV did not afford protection to mice suggested that the innate immune response would also be required to afford protection. Early studies indicated that Type I IFN elicited during early infection was a critical determinant in controlling the outcome of RVFV infection among rodents and non-human primates [46-49]. Subsequent studies confirmed these observations and also showed that virulent strain of RVFV inhibited IFN Type I and caused severe and fatal infection [50-52], but the attenuated MP-12 virus did not inhibit Type I IFN production, thus demonstrating that IFN played a critical role in protection during the early phase of RVFV infection [51-55]. Also, that IFN-α may afford protection was supported by a study that showed RVFV MP-12 vaccinated rhesus monkeys were protected against challenge with virulent RVFV because the monkeys secreted this cytokine within 12 hours of being challenged and did not develop disease [47]. Further evidence that IFN-α/β may have afforded protection to mice included the observation that the avirulent RVFV MP-12 vaccine was excellent inducer of early IFN-α/β production and that type 1 IFN peaked as early as 3 to 12 hours after the onset of infection with avirulent strains of RVFV virus [48,54]. Also, among RVFV-infected monkeys, the animals that had detectable levels of interferon at 12 hours after infection remained healthy, whereas those that did not have detectable serum interferon until 24-30 h after infection developed a clinical disease syndrome similar to human cases of RVF disease [47]. A more recent study showed that nAb was not detectable in mice on days 1- 4 following vaccination of mice with the MP-12, but 50% of the mice were protected from challenge with a lethal dose of virulent RVFV on day 2 PV and 100% were protected day 4, 5, 6 and 7 PV, thus suggesting a protective role for the innate and/or a cellular mediated immune response [56]. These findings demonstrated that the RVFV MP-12 vaccine elicited an innate protective immune response in mice during the first few days of PV, thus supporting a protective role for the innate immune response that functions in combination with both the humoral and cellular mediated immune response to protect animals during the early phase of RVFV infection in mice.
Our study design also precluded consideration of the well-documented protective role of RVFV-mediated cell-mediated immune response. A possible role of cellular mediated response during the early onset of the antiviral protective response was demonstrated by showing that a single dose of an RVFV replicon particle vaccine afforded protection to 60% of mice by day 1 PV and 100% protection to all mice by day 4 PV [57]. In that study, the immunization of mice with RVFV replicon particles upregulated antiviral genes, including IFN-β and other genes that play an important role in the initiation of cell-mediated and humoral immunity Other than eliciting a partially protective immune response by the RVFV Np, a study using A2-trangenic mice demonstrated that the RVFV Np protein could serve as a potent human T cell immunogen to elicit broad, immunodominant CD8+T cell responses that could be protective [58]. Although antiviral CD8+ T cells do not prevent infections, they promote the clearance of the virus by producing pro-inflammatory cytokines and direct killing of virus-infected cells, thus limiting virus dissemination and host morbidity [59]. Further evidence that the cellular immune response may have afforded protection to sheep early during RVFV infection was supported by the observation that the non-lethal response of non-human primates to RVFV infection was associated with early proliferation of CD4+ and CD8+ T cells and the early production of Th1 cytokine, including IFNγ [60]. IFN-γ has been shown to reduce viremia, and to prevent clinical signs in RVFV-infected rhesus monkeys [61]. These findings and the demonstrated critical role of T-cells, especially CD4+ T cells in eliciting strong antibody responses revealed the critical importance and benefit of a robust cellular response including effector memory T cells that secrete IFNγ within hours of re-exposure to antigen and these cells were detectable in some individuals many years after exposure [62,63]. As such, these findings suggested that vaccination with the MP-12 vaccine would have elicited a cellular-mediated immune response that could have afforded protection to mice against the challenge of a lethal dose of RVFV during early infection in this study.
A possible limitation of this study was the use of the MP-12 virus in the PRNT80 to quantify the titers of the antibody elicited in sheep by the MP-12 vaccine as well as to determine the protective efficacy of the antibody in mice. As a result, estimates of the nAb titers may have differed from the concentration that would have been detected if a pathogenic strain of RVFV had been used in the test. However, a previous experiment based on the PRNT80 cross-neutralization test showed that the neutralizing activity of the antibody elicited by the MP-12 and the virulent ZH501 strains of RVFV in sheep and nonhuman primates were comparable (Morrill JC, unpublished data). The most likely reason was because of the relatively low genetic diversity of approximately 4% and 1% at the nucleotide and protein-coding levels, respectively of RVFV isolates that comprised the seven known RVFV lineages [64]. As a result of the high degree of sequence conservation of the M segment genes that encode virion surface glycoproteins, antibodies elicited by a single strain of RVFV neutralized all circulating RVFV, implying that antibodies elicited by a single vaccine would protect against all lineages of the pathogenic strains of RVFV.
Conclusion
As described in this report, an alternative approach to estimating vaccine efficacy is to collect blood samples from vaccinated animals at intervals and passive transfer of sera samples to mice and then challenge the animals with virulent RVFV. In contrast to the classical challenge method, this passive antibody transfer procedure does not require large animal holding facilities, more animals can be used, and estimates of efficacy can be determined immediately or during the first week and subsequently at weekly or monthly intervals post-vaccination. While this passive transfer of antibody challenge method still requires the use of virulent strains of RVFV in a BSL-3+ laboratory, further studies are needed to develop an immune-competent murine model susceptible to the MP-12 virus that can be used in a BSL2 laboratory. The findings using this approach further confirm that nAb alone elicited by the MP-12 and the MP-12NSm-del vaccines afforded protection to animals against the virulent strain of the RVFV. The minimal protective nAb titers elicited in vaccinated sheep with only one dose of the MP-12 and MP-12NSm-del vaccines ranged from 10 to 20. Although the observation was for only one sheep, the results suggested that the innate and/or cellular immune response was needed to afford protection during the first week after vaccination. The findings offer a promising BALB/c mouse RVFV challenge model as a surrogate for evaluating the protective nAb response elicited by RVFV vaccines. Overall, the findings further supported the potential effectiveness of the MP-12 and MP-12NSm-del vaccine candidates for preventing RVF among humans and domestic ruminants.
The authors thank Dr. Heather Greenstone, Virology Branch, NIAID/NIH for her critical review of the manuscript.
Institutional review board statement
All animal procedures complied with USDA guidelines and were conducted at the AAALAC-accredited laboratory animal research facilities at Utah State University under protocol #10248 and approved by the Utah State University Institutional Animal Care and Use Committee on October 15, 2019
Author contributions
The following authors, D.M. Watts, B.B. Gowen, P.M. Palermo, K.W. Bailey, G.E. Bettinger, T.P. Monath, D.R. Smith, J.C. Morrill, J. Orbegozo, and J.L.B. Westover contributed substantially to the conception and design of the study. D.M. Watts, B.B. Gowen, P.M. Palermo, K.W. Bailey, J. Orbegozo, and J.L.B. Westover performed the study and collected, analyzed, and interpreted the data. D.M. Watts, B. B. Gowen, D. R. Smith, and P.M. Palermo prepared the manuscript, and all authors participated in the revision of the manuscript, agreed to be accountable for all aspects of the work, and approved the final version of this manuscript. As the leaders of the research program on Rift Valley fever, Phillip R. Pittman and all the other authors reviewed and complied with the journal policies detailed in the guide for authors.
Funding
This work was supported by contract HHSN272201700041/HHSN27200004/A11 from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Data availability statement
The datasets used and/or analyzed during this study are included as supplementary material and are available from the corresponding author upon reasonable request.
Disclaimer
This disclaimer is presented on-behalf of the co-author, DRS: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Defense, the Navy, or the U.S. Government. The Co-author, DRS is a military service member and federal employee of the U.S. Government. This work was prepared as part of her official duties. Title 17U.S.C. § 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. This disclaimer is presented on behalf of the Co-author DRS: Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by DOD. Research on human subjects was conducted in compliance with U.S. Department of Defense, federal, and state statutes and regulations relating to the protection of human subjects and adheres to principles identified in the Belmont Report (1979).
- Adams MJ, Lefkowitz EJ, King AMQ, Harrach B, Harrison RL, Knowles NJ, et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses. Arch Virol. 2017;162(10):2505-2538. Available from: https://doi.org/10.1007/s00705-017-3358-5
- McMillen CM, Hartman AL. Rift Valley fever in animals and humans: Current perspectives. Antiviral Res. 2018;156:29-37. Available from: https://doi.org/10.1016/j.antiviral.2018.05.009
- Peyre M, Chevalier V, Abdo-Salem S, Velthuis A, Antoine-Moussiaux N, Thiry E, et al. A systematic scoping study of the socio-economic impact of Rift Valley fever: research gaps and needs. Zoonoses Public Health. 2015;62(4):309-325. Available from: https://doi.org/10.1111/zph.12153
- Botros B, Omar A, Elian K, Mohamed G, Soliman A, Salib A, et al. Adverse response of non-indigenous cattle of European breeds to live attenuated Smithburn Rift Valley fever vaccine. J Med Virol. 2006;78(6):787-791. Available from: https://doi.org/10.1002/jmv.20624
- Kitandwe PK, McKay PF, Kaleebu P, Shattock RJ. An Overview of Rift Valley Fever Vaccine Development Strategies. Vaccines (Basel). 2022;10(11):1794. Available from: https://doi.org/10.3390/vaccines10111794
- Dungu B, Lubisi BA, Ikegami T. Rift Valley fever vaccines: current and future needs. Curr Opin Virol. 2018;29:8-15. Available from: https://doi.org/10.1016/j.coviro.2018.02.001
- Kamal SA. Observations on Rift Valley fever virus and vaccines in Egypt. Virol J. 2011;8:532. Available from: https://virologyj.biomedcentral.com/articles/10.1186/1743-422X-8-532
- Grobbelaar AA, Weyer J, Leman PA, Kemp A, Paweska JT, Swanepoel R. Molecular epidemiology of Rift Valley fever virus. Emerg Infect Dis. 2011;17(12):2270-2276. Available from: https://doi.org/10.3201/eid1712.111035
- Ikegami T. Rift Valley Fever vaccines: An overview of the safety and efficacy of the live-attenuated MP-12 vaccine candidate. Expert Rev Vaccines. 2017;16(6):601-611. Available from: https://doi.org/10.1080/14760584.2017.1321482
- Faburay B, LaBeaud AD, McVey DS, Wilson WC, Richt JA. Current Status of Rift Valley Fever Vaccine Development. Vaccines (Basel). 2017;5(3):29. Available from: https://doi.org/10.3390/vaccines5030029
- Caplen H, Peters CJ, Bishop DHL. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J Gen Virol. 1985;66(11):2271-2277. Available from: https://doi.org/10.1099/0022-1317-66-10-2271
- Saluzzo JF, Smith JF. Use of reassortant viruses to map attenuating and temperature-sensitive mutations of the Rift Valley fever virus MP-12 vaccine. Vaccine. 1990;8(5):369-375. Available from: https://doi.org/10.1016/0264-410x(90)90096-5
- Takehara K, Min MK, Battles JK, Sugiyama K, Emery VC, Dalrymple JM, et al. Identification of mutations in the mRNA of a candidate vaccine strain of Rift Valley fever virus. Virology. 1989;169(2):452-457. Available from: https://doi.org/10.1016/0042-6822(89)90171-2
- Vialat P, Muller R, Vu TH, Prehaud C, Bouloy M. Mapping of the mutations present in the genome of the Rift Valley fever virus attenuated MP12 strain and their putative role in attenuation. Virus Res. 1997;52(1):43-50. Available from: https://doi.org/10.1016/s0168-1702(97)00097-x
- Lihoradova OA, Indran SV, Kalveram B, Lokugamage N, Head JA, Gong B, et al. Characterization of Rift Valley Fever Virus MP-12 Strain Encoding NSs of Punta Toro Virus or Sandfly Fever Sicilian Virus. PLoS Negl Trop Dis. 2013;7(4):e2181. Available from: https://doi.org/10.1371/journal.pntd.0002181
- Won S, Ikegami T, Peters CJ, Makino S. NSm Protein of Rift Valley Fever Virus Suppresses Virus-Induced Apoptosis. J Virol. 2007;81(24):13335-13345. Available from: https://doi.org/10.1128/jvi.01238-07
- Gerrard SR, Nichol ST. Synthesis, proteolytic processing, and complex formation of N-terminally nested precursor proteins of the Rift Valley fever virus glycoproteins. Virology. 2007;357(1):124-133. Available from: https://doi.org/10.1016/j.virol.2006.08.002
- Kalveram B, Lihoradova O, Indran SV, Ikegami T. Using Reverse Genetics to Manipulate the NSs Gene of the Rift Valley Fever Virus MP-12 Strain to Improve Vaccine Safety and Efficacy. J Vis Exp. 2011;e3400. Available from: https://doi.org/10.3791/3400
- Adamson EK, Nyundo S, Rowland J, Palermo PM, Matiko MK, Bettinger GE, et al. Safety and Immunogenicity of Rift Valley Fever MP-12 and a Novel arMP-12ΔNSm21/384 Recombinant Vaccine Candidate in Native Breed of Black Head Sheep (Ovis aries) from Tanzania. J Vaccines Vaccin. 2018;9:4. Available from: https://www.researchgate.net/profile/Pedro-Palermo/publication/327714237_Safety_and_Immunogenicity_of_Rift_Valley_Fever_MP-12_and_a_Novel_arMP-12NSm21384_Recombinant_Vaccine_Candidate_in_Native_Breed_of_Black_Head_Sheep_Ovis_aries_from_Tanzania/links/5ca6d7734585157bd322f1ba/Safety-and-Immunogenicity-of-Rift-Valley-Fever-MP-12-and-a-Novel-arMP-12NSm21-384-Recombinant-Vaccine-Candidate-in-Native-Breed-of-Black-Head-Sheep-Ovis-aries-from-Tanzania.pdf?origin=journalDetail&_tp=eyJwYWdlIjoiam91cm5hbERldGFpbCJ9
- Nyundo S, Adamson EK, Rowland J, Palermo PM, Matiko MK, Bettinger GE, et al. Safety and immunogenicity of Rift Valley fever MP-12 and arMP-12ΔNSm21/384 vaccine candidates in goats (Capra aegagrus hircus) from Tanzania. Onderstepoort J Vet Res. 2019;86(1):e1-e8. Available from: https://doi.org/10.4102/ojvr.v86i1.1683
- Salekwa LP, Nyundo SB, Adamson EK, Matiko MK, Bettinger GE, Rowland JM, et al. Evaluation of the Immunogenicity of a Mutagenized Rift Valley Fever MP-12 and a Recombinant Rift Valley Fever arMP12ΔNsm21/384 Vaccine Candidates in Indigenous Species of Cattle, Sheep, and Goats in Tanzania. J Vaccines Vaccin. 2019;10:403. Available from: https://hdl.handle.net/10520/EJC-1463cf3b31
- Hunter P, Erasmus BJ, Vorster JH. Teratogenicity of a mutagenised Rift Valley fever virus (MVP 12) in sheep. Onderstepoort J Vet Res. 2002;69(1):95-98. Available from: https://pubmed.ncbi.nlm.nih.gov/12092782/
- Morrill JC, Laughlin RC, Lokugamage N, Pugh R, Sbrana E, Weise WJ, et al. Safety and immunogenicity of recombinant Rift Valley fever MP-12 vaccine candidates in sheep. Vaccine. 2013;31(4):559-565. Available from: https://doi.org/10.1016/j.vaccine.2012.10.118
- Morrill JC, Laughlin RC, Lokugamage N, Wu J, Pugh R, Kanani P, et al. Immunogenicity of a recombinant Rift Valley fever MP-12-NSm deletion vaccine candidate in calves. Vaccine. 2013;31(48):4988-4994. Available from: https://doi.org/10.1016/j.vaccine.2013.08.003
- Weingartl HM, Nfon CK, Zhang S, Marszal P, Wilson WC, Morrill JC, et al. Efficacy of a recombinant Rift Valley fever virus MP-12 with NSm deletion as a vaccine candidate in sheep. Vaccine. 2014;32(20):2345-2349. Available from: https://doi.org/10.1016/j.vaccine.2013.12.064
- Boumart Z, Daouam S, Bamouh Z, Jazouli M, Otadlaoui KO, Dungu B, et al. Safety and immunogenicity of a live attenuated Rift Valley Fever recombinant arMP 12ΔNSm21/384 vaccine candidate for sheep, goats and calves. Vaccine. 2019;37(12):1642-1650. Available from: https://doi.org/10.1016/j.vaccine.2019.01.067
- Nyundo S, Adamson EK, Rowland J, Salekwa L, Palermo PM, Matiko MK, et al. Assessment of the Immunogenicity of a Novel Live Recombinant Rift Valley Fever arMP-12ΔNSm21/384 Vaccine Candidate Following Intranasal Vaccination of Goats, Sheep and Calves in Tanzania. J Vaccines Vaccin. 2020;11:430. Available from: https://www.researchgate.net/profile/Pedro-Palermo/publication/346570875_Assessment_of_the_Immunogenicity_of_a_Novel_Live_Recombinant_Rift_Valley_Fever_arMP-_12DNSm21384_Vaccine_Candidate_Following_Intranasal_Vaccination_of_Goats_Sheep_and_Calves_in_Tanzania/links/5fd9a3ba45851553a0bd7268/Assessment-of-the-Immunogenicity-of-a-Novel-Live-Recombinant-Rift-Valley-Fever-arMP-12DNSm21-384-Vaccine-Candidate-Following-Intranasal-Vaccination-of-Goats-Sheep-and-Calves-in-Tanzania.pdf
- Bamouh Z, Boumart Z, Elhayane M, Tadlaoui KO, Bettinger G, Watts DM, et al. Immunogenicity monitoring of the recombinant Rift Valley fever arMP-12 ΔNSm21/384 vaccine in sheep. Vaccine and Vaccination. Vaccines Vaccin. 2022;13:481. Available from: https://www.mci-santeanimale.com/wp-content/uploads/2022/05/9-RVF-MP12-2022.pdf
- Boumart Z, Bamouh Z, Hamdi J, Safini N, Tadlaoui KO, Bettinger G, et al. Safety and immunogenicity of the Rift Valley fever arMP-12 ΔNSm21/384 candidate vaccine in pregnant ewes. Vaccine. 2020;38(44):100070. Available from: https://doi.org/10.1016%2Fj.jvacx.2020.100070
- Hickerson BT, Westover JB, Van Wettere AJ, Rigas JD, Miao J, Conrad BL, et al. Pathogenesis of Rift Valley Fever Virus Aerosol Infection in STAT2 Knockout Hamsters. Viruses. 2018;10(11):651. Available from: https://doi.org/10.3390/v10110651
- Morrill JC, Jennings GB, Caplen H, Turell MJ, Johnson AJ, Peters CJ. Pathogenicity and immunogenicity of a mutagen-attenuated Rift Valley fever virus immunogen in pregnant ewes. Am J Vet Res. 1987;48(7):1042-1047. Available from: https://pubmed.ncbi.nlm.nih.gov/3631685/
- Morrill JC, Carpenter L, Taylor D, Ramsburg HH, Quance J, Peters CJ. Further evaluation of a mutagen attenuated Rift Valley fever vaccine in sheep. Vaccine. 1991;9(1):35-41. Available from: https://doi.org/10.1016/0264-410x(91)90314-v
- Dungu B, Louw I, Lubisi A, Hunter P, von Teichman BF, Bouloy M. Evaluation of the efficacy and safety of the Rift Valley fever clone 13 vaccine in sheep. Vaccine. 2010;28(19):4581-4587. Available from: https://doi.org/10.1016/j.vaccine.2010.04.085
- Kortekaas J, Antonis AF, Kant J, Vloet RP, Vogel A, Oreshkova N, et al. Efficacy of three candidate Rift Valley fever vaccines in sheep. Vaccine. 2012;30(24):3423-3429. Available from: https://doi.org/10.1016/j.vaccine.2012.03.027
- Smith DR, Johnston SC, Piper A, Botto M, Donnelly G, Shamblin J, et al. Attenuation and efficacy of live-attenuated Rift Valley fever virus vaccine candidates in non-human primates. PLoS Negl Trop Dis. 2018 May 9;12(5): e0006474. Available from: https://doi.org/10.1371/journal.pntd.0006474
- Smith DR, Steele KE, Shamblin J, Honko A, Johnson J, Reed C, et al. The pathogenesis of Rift Valley fever virus in the mouse model. Virology. 2010;407(2):256-267. Available from: https://doi.org/10.1016/j.virol.2010.08.016
- Watts DM, Westover JLB, Palermo PM, Bailey KW, Morrill JC, Bettinger GE, et al. Estimation of the Minimal Rift Valley Fever Virus Protective Neutralizing Antibody Titer in Human Volunteers Immunized with MP-12 Vaccine Based on Protection in a Mouse Model of Disease. Am J Trop Med Hyg. 2022;107(6):1091-1098. Available from: https://doi.org/10.4269/ajtmh.22-0356
- Niklasson BS, Meadors GF, Peters CJ. Active and passive immunization against Rift Valley fever virus infection in Syrian hamsters. Acta Pathologica, Microbiologica, et Immunologica Scandinavica. 1984;92(3):197-200. Available from: https://doi.org/10.1111/j.1699-0463.1984.tb00074.x
- Paweska JT, Smith SJ, Wright IM, Williams R, Cohen AS, Van Dijk AA, et al. Indirect enzyme-linked immunosorbent assay for the detection of antibodies against Rift Valley fever virus in domestic and wild ruminant sera. Onderstepoort J Vet Res. 2003;70(1):49-64. Available from: https://pubmed.ncbi.nlm.nih.gov/12825681/
- Fafetine JM, Tijhaar E, Paweska JT, Neves LC, Hendriks J, Swanepoel R, et al. Cloning and expression of Rift Valley fever virus nucleocapsid (N) protein and evaluation of an N-protein-based indirect ELISA for the detection of specific IgG and IgM antibodies in domestic ruminants. Vet Microbiol. 2007;121(1-2):29-38. Available from: https://doi.org/10.1016/j.vetmic.2006.11.008
- Lagerqvist N, Naslund J, Lundkvist A, Bouloy M, Ahlm C, Bucht G. Characterization of immune responses and protective efficacy in mice after immunization with Rift Valley Fever virus cDNA constructs. Virol J. 2009;6:6. Available from: https://doi.org/10.1186/1743-422x-6-6
- Boshra H, Lorenzo G, Rodriguez F, Brun A. A DNA vaccine encoding ubiquitinated Rift Valley fever virus nucleoprotein provides consistent immunity and protects IFNAR(-/-) mice upon lethal virus challenge. Vaccine. 2011 Jun;29(23):4469-4475. Available from: https://doi.org/10.1016/j.vaccine.2011.04.043
- Jansen van Vuren P, Tiemessen CT, Paweska JT. Anti-nucleocapsid protein immune responses counteract pathogenic effects of Rift Valley fever virus infection in mice. PLoS One. 2011;6(9): e25027. Available from: https://doi.org/10.1371/journal.pone.0025027
- López-Gil E, Moreno S, Ortego J, Borrego B, Lorenzo G, Brun A. MVA Vectored Vaccines Encoding Rift Valley Fever Virus Glycoproteins Protect Mice against Lethal Challenge in the Absence of Neutralizing Antibody Responses. Vaccines. 2020;8(1):82. Available from: https://doi.org/10.3390/vaccines8010082
- Gutjahr B, Keller M, Rissmann M, Von Arnim F, Jackel S, Reiche S, et al. Two monoclonal antibodies against glycoprotein Gn protect mice from Rift Valley Fever challenge by cooperative effects. PLoS Negl Trop Dis. 2020;14(3): e0008143. Available from: https://doi.org/10.1371/journal.pntd.0008143
- Anderson GW Jr, Peters CJ. Viral determinants of virulence for Rift Valley fever (RVF) in rats. Microb Pathog. 1988;5(3):241-250. https://doi.org/10.1016/0882-4010(88)90096-4
- Morrill JC, Jennings GB, Cosgriff TM, Gibbs PH, Peters CJ. Prevention of Rift Valley fever in rhesus monkeys with interferon-alpha. Rev Infect Dis. 1989;11 Suppl 4: S815-25. https://doi.org/10.1093/clinids/11.supplement_4.s815
- Morrill JC, Jennings GB, Johnson AJ, Cosgriff TM, Gibbs PH, Peters CJ. Pathogenesis of Rift Valley fever in rhesus monkeys: role of interferon response. Arch Virol. 1990;110(3):195-212. Available from: https://doi.org/10.1007/bf01311288
- do Valle TZ, Billecocq A, Guillemot L, Alberts R, Gommet C, Geffers R, et al. A new mouse model reveals a critical role for host innate immunity in resistance to Rift Valley fever. J Immunol. 2010 Nov 15;185(10):6146-6156. Available from: https://doi.org/10.4049/jimmunol.1000949
- Habjan M, Pichlmair A, Elliott RM, Overby AK, Glatter T, Gstaiger M, et al. NSs protein of Rift Valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J Virol. 2009 May;83(9):4365-4375. Available from: https://doi.org/10.1128/jvi.02148-08
- Vialat P, Billecocq A, Kohl A, Bouloy M. The S segment of Rift Valley fever phlebovirus (Bunyaviridae) carries determinants for attenuation and virulence in mice. J Virol. 2000;74(4):1538-1543. Available from: https://doi.org/10.1128/jvi.74.3.1538-1543.2000
- Billecocq A, Spiegel M, Vialat P, Kohl A, Weber F, Bouloy M, et al. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J Virol. 2004 Sep;78(18):9798-9806. Available from: https://doi.org/10.1128/jvi.78.18.9798-9806.2004
- Bouloy M, Janzen C, Vialat P, Khun H, Pavlovic J, Huerre M, et al. Genetic evidence for an interferon-antagonistic function of Rift Valley fever virus nonstructural protein NSs. J Virol. 2001;75(3):1371-1377. Available from: https://doi.org/10.1128/jvi.75.3.1371-1377.2001
- Higashihara M, Koyama H, Igarashi Y. Induction of virus-inhibiting factor or interferon in mice by strains with different virulence of Rift Valley fever virus. Kitasato Arch Exp Med. 1972;45(1):33-43. Available from: https://pubmed.ncbi.nlm.nih.gov/4632205/
- Billecocq A, Vialat P, Spiegel M, Weber F, Haller O, Bouloy M. The RVFV NSs antagonizes the antiviral response of the infected cells: analysis of the first steps of the activation of interferon production. Abstracts of the XII International Conference on Negative Strand Viruses, Pisa, Italy, June 14-19, 2003; 137-156.
- Morrill JC, Peters CJ, Bettinger GE, Palermo PM, Smith DR, Watts DM. Rift Valley fever MP-12 vaccine elicits an early protective immune response in mice. Vaccine. 2022;40(49):7255-7261. Available from: https://doi.org/10.1016/j.vaccine.2022.10.062
- Dodd KA, Bird BH, Metcalfe MG, Nichol ST, Albariño CG. Single-dose immunization with virus replicon particles confers rapid robust protection against Rift Valley fever virus challenge. J Virol. 2012;86(7):4204-4212. Available from: https://doi.org/10.1128/jvi.07104-11
- Xu W, Watts DM, Costanzo MC, Tang X, Venegas LA, Jiao F, et al. The nucleocapsid protein of Rift Valley fever virus is a potent human CD8+ T cell antigen and elicits memory responses. PLoS One. 2013;8(3): e59210. Available from: https://doi.org/10.1371/journal.pone.0059210
- Dodd KA, McElroy AK, Jones ME, Nichol ST, Spiropoulou CF. Rift Valley fever virus clearance and protection from neurologic disease are dependent on CD4+ T cell and virus-specific antibody responses. J Virol. 2013 Jun;87(11):6161-6171. Available from: https://doi.org/10.1128/jvi.00337-13
- Wonderlich ER, Caroline AL, McMillen CM, Walters AW, Reed DS, Barratt-Boyes SM, et al. Peripheral blood biomarkers of disease outcome in a monkey model of Rift Valley fever encephalitis. J Virol. 2018 Jan 17;92(3): e01662-17. Available from: https://doi.org/10.1128/jvi.01662-17
- Morrill JC, Czarniecki CW, Peters CJ. Recombinant human interferon-gamma modulates Rift Valley fever virus infection in the rhesus monkey. J Interferon Res. 1991;11(5):297-304. Available from: https://doi.org/10.1089/jir.1991.11.297
- Calarota SA, Baldanti F. Enumeration and characterization of human memory T cells by enzyme-linked immunospot assays. Clin Dev Immunol. 2013;637649. Available from: https://doi.org/10.1155/2013/637649
- Harmon JR, Barbeau DJ, Nichol ST, Spiropoulou CF, McElroy AK. Rift Valley fever virus vaccination induces long-lived, antigen-specific human T-cell responses. npj Vaccines. 2020;5(1):17. Available from: https://doi.org/10.1038/s41541-020-0166-9
- Bird BH, Khristova ML, Rollin PE, Ksiazek TG, Nichol ST. Complete genome analysis of 33 ecologically and biologically diverse Rift Valley fever virus strains reveals widespread virus movement and low genetic diversity due to recent common ancestry. J Virol. 2007;81(6):2805-2816. Available from: https://doi.org/10.1128/jvi.02095-06
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
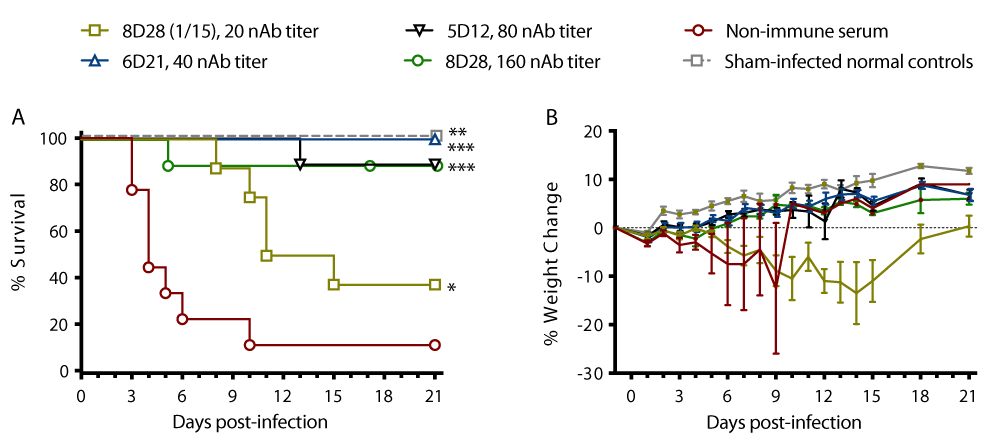


 Save to Mendeley
Save to Mendeley
