Global Journal of Zoology
Longitudinal spatial distribution of Aedes aegypti (Diptera: Culicidae) during the yellow fever epidemic in Angola, 2016
María del Carmen Marquetti Fernández1*, Troco Arletty2, Cani P2 and Yoenys Hidalgo Flores3
2National Malaria Control Program, Angola, Cuba
3Cuban Control Program Anti-Larval Malaria Vectors with Biolarvicides in Angola, Cuba
Cite this as
Marquetti Fernández MDC, Arletty T, Cani P, Flores YH (2018) Longitudinal spatial distribution of Aedes aegypti (Diptera: Culicidae) during the yellow fever epidemic in Angola, 2016. Glob J Zool 4(1): 001-006. DOI: 10.17352/gjz.000011Background and aims: Angola is one of the countries included in the endemic areas of yellow fever transmission in Africa. The objective of this study was to obtain information about the longitudinal spatial distribution and the mainly breeding sites of Ae. aegypti in Angola during the yellow fever epidemic in Angola during 2016.
Methods: Angola is located in the western region of Southern Africa. The country limited by the Atlantic Ocean in the west, in the north with the Republic of Congo and the Democratic Republic of Congo, in the eastern with the Republic of Zambia, and in the south with the Republic of Namibia. It is divided in 18 provinces. The sampling was carried out in 51 municipalities distributed in all provinces, in villages and neighborhoods with suspicious and confirmed cases of yellow fever during February- October 2016. The number of houses inspected was 20 only one time during the months for to determine presence or absence of Aedes aegypti.
Results: Ae. aegypti presence was observed in 16/18 (88.8%) of the province 42/51 (82.3%) of the municipalities and 241/277 (87%) of the villages or neighborhoods sampled. Fifteen news municipalities that represent new records for Ae. aegypti presence in Angola were notified. Ae. aegypti larvae were collected in 22 types of containers, mainly in water storage containers followed by plants in water and potted, artificial miscellaneous containers and used car tires in Luanda.
Conclusions: This work expanded the knowledge on distribution and breeding sites of Ae. aegypti in Angola.
Introduction
Aedes aegypti is native from Africa and spread to other tropical countries in the 17th and 18th centuries [1-3]. Other species of mosquitoes of the genus Aedes including Aedes albopictus, Aedes africanus, Aedes metallicus and Aedes luteocephalus are registered in the African continent and are considered potential vectors of dengue, yellow fever , chikungunya, zika and other arbovirus [4-6]. Ae. aegypti was registered in Angola in 1903 [7].
Angola is one of the countries included in the endemic areas of yellow fever transmission in Africa and where there have been outbreaks of this disease in 1971 and in 1988 in its capital Luanda, as well as, sporadic cases identified through the surveillance system. Prior to these outbreaks, serological samples were taken for antibodies to arboviruses where it was shown that the virus was active in some areas of the south and southeast of the country where the ecological conditions are favorable, but note that if there were cases, these ones were not notified due to the absence of facilities for diagnosis previous and during the decade of the 60’s of the last 20th century [8-12].
Yellow fever virus is an RNA virus that belongs to the genus Flavivirus. It is related to West Nile, St. Louis encephalitis, and Japanese encephalitis viruses. This virus is transmitted to humans by mosquitoes. Among the vector species in Angola are Aedes africanus responsible for the maintenance of sylvatic transmission; Aedes metallicus in rural habitats in the northeast of the country; Aedes metallicus and Aedes vittatus responsible in the southwest and in the central west Aedes luteocephalus [13]. In urban area such as Luanda Aedes aegypti is present with a strong association with humans and is the responsible for the urban transmission [14, 15]. In another hands dengue activity has been reported sporadically in the country too [16].
In general, to literature on mosquito fauna in Angola and, in particular, to the presence of genus Aedes are reflected in several researchers by various authors [17-20], During the second half of the XX century 48 mosquito’s species were registered. Ae. aegypti presence was summarized in several localities of the country during these studies [13]. There is only scanty data available about Ae. aegypti during this century in Angola [14,21]. For this reason the objective of this study was to obtain information about the spatial distribution and the mainly breeding sites of Ae. aegypti in Angola during the yellow fever epidemic registered in Angola during 2016.
Materials and Methods
Brief description of the study area
The Republic of Angola is located in the western region of Southern Africa, with a surface area of 1 246 700 km2. The western of the country is limited by the Atlantic Ocean, in the north limited with borders, with the Republic of Congo and the Democratic Republic of Congo, in the eastern with the Republic of Zambia, and in the south with the Republic of Namibia figure 1. Angola is divided in 18 Provinces: Bengo, Benguela, Bie, Cabinda, Cunene, Huila, Huambo, Cuando Cubango, Cuanza Norte, Cuanza Sul, Lunda Norte, Lunda Sul, Luanda (capital of the country), Malange, Moxico, Namibe, Uige and Zaire. The country is rich, especially in minerals diamonds, iron, manganese, among others, oil and agriculture stands out as a producer of coffee and corn. The climate has two seasons raining between September to middle of the May and dry season to June to August [22]. Angola population was estimated at 25 789 024 inhabitants (given by the last National Census conducted on May 15-31, 2014 National Institute of Statistics).
Entomological sampling, execution time and identification of samples
The sampling was carried out in 51 municipalities distributed in 18 provinces, in villages and neighborhoods with suspicious and confirmed cases of yellow fever in the period of February- October 2016. Sample collections of immature stages of mosquitoes were carried out in the dwellings with the presence of cases and in the surroundings of the same, not moving more than 100 meters in all directions from the house subject to the investigation, the number of houses and patios reviewed was 20 in each visited locality only one time. All deposits with water were checked for the presence of the vector. The larvae were collected using a dropper, placed in flasks with 70% alcohol labeled with the data of the place of collection, date and type of deposit. For the taxonomic classification of the mosquito samples, larvae III and IV stage were selected only. We reviewed works related to the taxonomy and systematics of these insects [13,23-25], but chose to use the morphological keys for presenting relevant information about the Aedes genus in Angola. It is convenient to clarify that taxonomic works carried out in the last years make a relocation of the Aedes Meigen species, 1818 in other genera [26], however, we decided in this work not to use the classification proposed by these authors.
Results
The total of provinces municipalities and neighborhoods sampled are shown in table 1, resulting with Ae. aegypti presence in 16/18 (88.8%) of the province 42/51 (82.3%) of the municipalities and 241/277 (87%) of the villages or neighborhoods. Ae. aegypti was recorded during this study in the 7 municipalities that make up Luanda province in 2016 (Belas, Cacuaco, Viana, Icolo de Bengo, Quissama, Cazenga and Luanda (municipality with the same name as the province and composed of the districts of Ingombota, Rangel, Samba, Sambizanga, Maianga and Kilamba Kiaxi) and 174/182 (95.6%) of the neighborhoods sampled.
A total of 15 news municipalities that represent new records for Ae, aegypti presence in Angola during 2016 are showed in table 2. Luanda was not included in this table because it presented several modifications in its administrative division in the two periods that are compared and Bie for not being sampled in 1973 while Bengo for not existing as a province in 1973. A total of 512 Ae. aegypti larvae were collected in a total of 65 breeding sites divided into 12 types, where water storage containers (buckets, tanks, cisterns, drums, basins and washing container) contributed to the major Ae. aegypti presence 44/65 (67%) followed by miscellaneous containers 8/65 (12.3%); used car tires 7/65(10.7%) and cement blocks 6/65 (10%) table 3.
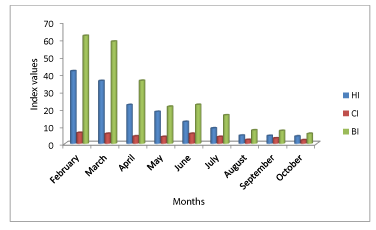
A total of 32 760 households were surveyed in all Luanda municipalities during February to October of which 9 711 (29.6%) were positive to Ae. aegypti mosquito larvae. During the study period House index oscillated between 41.9 in February to 4.3 in October. The total of container inspected was 156 789 of these 4 478 (2.8%) were positive to larvae. Container index oscillated between 6.3 in February to 2.1 in October. Breteau index oscillated between 62.2 in February to 5.8 in October figure 2. The average household container with water was 4.7. Ae. aegypti was collected in 22 types of containers, where water storage containers 3 501/4478(78.1%) contributed to the major Ae. aegypti presence in Luanda followed by plants in water and potted 346/4478 (7.7%); artificial miscellaneous containers (tins, jars, bottles., etc 335/4478 (7.4%) and used car tires 296/4478 (6.8%).
Aedes aegypti was found associated with larvae of Culex sp and Anopheles sp. at the breeding sites in several municipalities belonging Luanda, Cuanza Sul, Benguela and Bengo provinces.
Discussion
The greater distribution of Ae. aegypti was observed in the provinces located in the central west, mainly in the coastal areas, which corresponds to the distribution of suspicious cases of yellow fever reported in the country (until June 27 there were a total of 3 496 suspicious cases of these 2 449 belonged to the coastal provinces for 70%, 903 to the provinces of Huila, Huambo, Uige, K. North, Cunene and Malange for 25.8%, adding up in total for the west center area 95.8% of the total of suspected cases) [28]. The coastal strip in the western region where is located Luanda province is characterized by having the highest average temperatures and the lowest altitude with respect to sea level, fundamental factors for the distribution of the vector.
During the sampling carried out in the province of Luanda, Ae. aegypti in the 7 municipalities that compose it even at the end of May and the month of June belonging to the dry season (May-September) in which the samplings were carried out due to deficiencies with the supply and distribution of water in most of its municipalities which leads to the storage of this liquid in various deposits used for this function, resulting in the most positive presence of the vector, coinciding with previous results obtained in Luanda and other countries of the world [28-32].
It should be noted that part of the sites with the presence of Ae. aegypti found in the provinces sampled were in the exteriors and roofs of houses (used car tires, cement blocks, plants in water and potted) in the last months of the rainy season (February-April) and early the dry season (end of May) showing a reduction of the positivity in the samplings of the month of June in these types of breeding sites. These results suggest directing social mobilization to the cover of the deposits, mainly in areas where the water supply is due to laminar and unstable water and to the sanitation of yards and roofs of the houses before and during the rainy season.
Aedes aegypti was not found during the survey in the localities sampled in the provinces of Lunda Norte and Cuando Cubango which does not imply that it is not present, since in reports made before 1973 it was reported in Dundo, Lunda Norte and in Menongue, Cuando Cubango. This absence could be due to the reduce number of houses that were inspected in search of the vector and that these provinces were visited already at the end of June where many possible deposits of the vector’s breeding sites were dry.
Conclusions
This work expanded the knowledge on distribution and breeding sites of Ae. aegypti in Angola with 15 new municipalities. On the other hand, the authors recommended first to extend the samplings of search of presence of the vector in the province of Bie and to repeat samplings in the provinces of Malange, Namibe and Lunda Norte in the rainy season. And second to reinforce the monitoring and surveillance of Ae. aegypti in the country because in the last years the arbovirus diseases have emerged and remerged dramatically in the world including African continent.
The authors wish thanks to Ministry of Health and all Heads of Health at the provinces, municipalities and villages or neighborhoods levels in Angola; Angolan brigades vector control staff; Cuban specialists belonging to Control Program Anti-Larval Against Malaria (LABIOFAM) and the population in general for his contribution to the realization of this work.
- Halstead SB Dengue (2008) overview and history. In: Halstead SB, editor. Dengue. London: Imperial College Press. 1–28.
- Brown JE, McBride CS, Johnson P, Ritchie S, Paupy C, et al. (2011) Worldwide patterns of genetic differentiation imply multiple ‘domestications’ of Aedes aegypti, a major vector of human diseases. Proceedings. Biological Sciences/The Royal Society 278: 2446–2454. Link: http://bit.ly/2LU6mk4
- Brown JE, Evans BR, Zheng W, Obas V, Barrera-Martinez L, et al. (2014) Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution 68: 514–525. Link: http://bit.ly/2XJuVaB
- Jentes ES, Poumerol G, Gershman MD, Hill DR, Lemarchand J, et al. (2011) Informal WHO Working Group on Geographic Risk for Yellow Fever The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the informal WHO Working Group on Geographic Risk for Yellow Fever. The Lancet. Infectious Diseases 11: 622–632. Link: http://bit.ly/2Yc8tpQ
- Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, De Lamballerie X (2014) Chikungunya in the Americas. Lancet 383: 514. Link: http://bit.ly/2LU780s
- Simmons CP, Farrar JJ, Chau NVV, Wills B (2012) Dengue. The New England Journal of Medicine 366: 1423–1432. Link: http://bit.ly/2Yc8z0G
- Roque AB (1903) Contribuição para o estudo da malaria e dos mosquitos de Angola. A Med Contemp Lisboa.
- Mora AD (1932) Yellow fever in Angola. Bull Trim Org Hyg 2: 56-59.
- Cambournac FJC (1954) Yellow fever in Angola and the islands of São Tomé and Principe. Bull World Health Organ 11: 504-507. Link: http://bit.ly/2Lqvgbu
- Cambournac FJC, Pinto MR, Jang J (1962) Note on the reservation existence of virus yellow fever in the south-eastern region of Angola. An Inst Med Trop 19: 15-19.
- Ribeiro H (1973) Entomological studies during the 1971 yellow fever epidemic of Luanda, Angola. Mosquito News 4: 568-574. Link: http://bit.ly/30ySQXd
- Ribeiro H (1971) Entomologia da epidemia de febre-amarela de Luanda, em 1971. Revista Médica de Angola 13: 67-91.
- Ribeiro H, Da Cunha Ramos H (1973) Research on the mosquitoes of Angola. VIII- The genus Aedes Meigen, 1818 (Diptera:Culicidae). Check-list with new record, keys to females and larvae, distribution and taxonomic and bioecological notes. Separata dos Anais do Instituto de Higiene e Medicina tropical. 1(1/4, Janeiro/Dezembro.
- Marquetti Fernández MC, Hidalgo Flores Y, Lamothe Nuviola D (2017) Spatial Distribution and Mainly Breeding Sites of Aedes aegypti (Diptera:Culicidae) in Luanda, Angola, Ann Community Med Pract 3. Link: http://bit.ly/2xS3Oec
- WHO (2016) Report Situation Yellow fever in Angola. http://bit.ly/2GhsTUj
- MMWR (2013) Ongoing Dengue Epidemic-Angola 62: 504-507. Link: https://bit.ly/2XPmPxh
- Colaço ATF (1952) Contribuição para o conhecimento dos Culicidae de Angola (Luanda, Sambo e Nova Lisboa). An Inst Med Trop Lisboa 9: 511-516. http://bit.ly/2Los5RK
- Gândara AF (1956) Subsidio para o estudo dos Culicidae (Diptera) de Angola. An Inst Med Trop Lisboa 13: 387-418.
- Gândara AF (1956) Contribution pour la connaissance des Culicidae d'Angola. Proc Tenth Intern Congr Entomol 3: 675-679.
- Ribeiro H, Mexia TJ (1966) Research on the mosquitoes of Angola (Diptera:Culicidae) III. A culicine survey in the Lobito-Catumbela region. An Inst Med Trop Lisboa 23: 167-182. Link: http://bit.ly/2LV7QKV
- Marquetti Fernández MC, Granado Labrada E, Troco A, Labañino Romero I, Cani P, et al. (2018) Impact of Vector Control Activities during the Yellow Fever Epidemic in Luanda, Angola, 2016. Ann Community Med Pract 4: 1030-1034.
- Ministério da Saúde (2014) Plano nacional de Desenvolvimento Sanitário em Angola 2012-2025. MINSA 116. Link: http://bit.ly/2YXaNy8
- González R (2006) Culícidos de Cuba. Editorial Científico Técnica. Book Ref. 2008
- Rueda LM (2004) Pictorial keys for the identification of mosquitoes (Diptera:Culicidae) associated with dengue virus transmission. Zootaxa 589. http://bit.ly/2XV49Y7
- Worth CB, Paterson HE (1961) Culicine mosquitoes in Southern Africa. Rev Ent Moç 4: 65-80. Link: http://bit.ly/2XIrCk1
- Reinert JF, Harbach RE, Kitching IJ (2009) Phylogeny and classification of tribe Aedini (Diptera: Culicidae) Zoological Journal of the Linnean Society 157: 700–794. Link: http://bit.ly/2ShBc7w
- WHO (2016) Report Situation Yellow fever, Angola Mayo. Link: http://bit.ly/2GgajvE
- Harun NN, Francis MM, Bryson AN, Peter SM, Mbakaya JO, et al. (2017) Characterization and productivity profiles of Aedes aegypti (L.) breeding habitats across rural and urban landscapes in western and coastal Kenya. Parasit Vectors 10: 331 Link: http://bit.ly/2Y6RYLH
- Espinosa M, Weinberg D, Rotela CH, Abril M, Scayuzzo CM, et al. (2016) Temporal Dynamics and Spatial Patterns of Aedes aegypti Breeding Sites, in the Context of a Dengue Control Program in Tartagal (Salta Province, Argentina). PLoS Negl Trop Dis 10: e0004621. Link: http://bit.ly/2NZTg7z
- Codeço CT, Arthur WSL, Araújo SC, Lima JBP, Maciel-de-Freitas R, et al. (2015) Surveillance of Aedes aegypti: Comparisonof House Index with four alternative traps, PLOS Neglected Tropical Diseases 9: e0003475. Link: http://bit.ly/2XR87Rv
- Marquetti MC, Fuster CA, Estévez G, Somarriba L (2011) Estudio descriptivo de la distribución y positividad larvaria de Aedes aegypti (Diptera:Culicidae) en Haití. Rev Biomédica 22: 77-84. Link: http://bit.ly/2XJNWK1
- Parfait L, Quattara E, Sangaré I, Namountougou M, Hien A, et al. (2019) Surveys of arboviruses vectors in four cities stretching along a railway transect of Burkina Faso: Risk transmission and insecticide susceptibility status of potential vectors. Frontiers in Veterinary Science 6: 140. Link: https://bit.ly/2XIZXiV
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
