Global Journal of Zoology
Centrobolus silvanus dimorphism based on tergite width
Mark Cooper*
Cite this as
Cooper M (2018) Centrobolus silvanus dimorphism based on tergite width. Glob J Zool 3(1): 003-005. DOI: 10.17352/gjz.000010The forest genus Centrobolus of diplopoda belonging to the Order Spirobolida is distributed along the eastern coast of southern Africa. Sexual size dimorphism (SSD) can be explained as sexual selection and fecundity selection. Width and length were analysed in Centrobolus to derive SSD in 22 species. Width size of C. silvanus collected in South Africa was calculated as 47.5 mm (n=8). Male width was 43 mm (μ ± σ; n=1) and female width 54 mm (μ ± σ; n=1). An analysis from data presently available showed average SSD for C. silvanus was 1.25581395 differing from 1 (t=1.52753, p=0.085235; n=6). C. silvanus dimorphism was based on a 11 mm difference in horizontal tergite width. Sexual dimorphism appeared as in C. inscriptus female width which was positively related to copulation duration. Keywords. C. silvanus, horizontal tergite width.
Introduction
Size differences of diplopods correlate with factors such as color, sexes, species, urbanisation and water relations [1,2]. SSD has consequences for outcomes of sexual encounters in diplopod mating [3-5]. The allometry of SSD involves the detection of a relationship between body size and SSD and is known as Rensch’s rule which may be explained as sexual selection and fecundity selection [6,7]. This allometric rule predicts SSD is negatively correlated with body size [8-10]. The forest genus of diplopods belonging to the Order Spirobolida found along the eastern coast of southern Africa was the subject of this study [11,17]. Length, width and ring data are known in 22 species (Centrobolus albitarsus, C. anulatus, C. decoratus, C. digrammus, C. dubius, C. fulgidus, C. immaculatus, C. inscriptus, C. inyanganus, C. lawrencei, C. lugubris, C. promontorius, C. pusillus, C. richardi, C. ruber, C. rugulosus, C. sagatinus, C. silvanus, C. titanophilus, C. transvaalicus, C. tricolor, and C. vastus). The revision of the genus Centrobolus (Cook, 1897) was part of this data [12-15]. SSD in these forest diplopods have been understood as size using Centrobolus to test body size relationships and the trend of SSD has been calculated for Centrobolus [2]. The present study re-illustrates the trend of SSD for the genus Centrobolus and shows the sizes of C. silvanus relative to 21 of congenerics in order to express how species do not follow the trend of Rensch’s rule [16].
Materials and Methods
One factor was analysed from Centrobolus silvanus: (1) horizontal tergite width (mm) [15,17]. C. silvanus (Attems) were collected from Kentani District and Knysna, South Africa. SSD was calculated based on the volumes for 22 species in the genus Centrobolus [16,18] and included data for C. richardi.
Statistical analysis
The basic descriptive figures of horizontal tergite width were analysed. The average width was obtained for 2 individuals of C. silvanus. Size was based on dorsal tergite width. SSD was the average female size divided by average male size and converted into a SSD ratio. Allometry for SSD was based on a model where male size = α (female)β. A linear regression was tested the relationship between sexual sizes at https://www.socscistatistics.com. SSD was compared to 1 using a t-test for 2 dependent means at https://www.socscistatistics.com/tests/studentttest/Default2.aspx.
Results
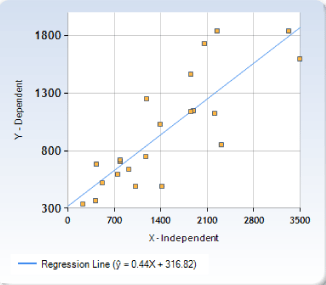
Sizes were estimated in the following 22 taxa: Centrobolus albitarsus, C. anulatus, C. decoratus, C. digrammus, C. dubius, C. fulgidus, C. immaculatus, C. inscriptus, C. inyanganus, C. lawrencei, C. lugubris, C. promontorius, C. pusillus, C. richardi, C. ruber, C. rugulosus, C. sagatinus, C. silvanus, C. titanophilus, C. transvaalicus, C. tricolor, and C. vastus. SSD is shown in figure 1. For the data, the regression equation for Y was: ŷ = 0.44407X + 316.81644. The quantitative resolution of the rule for Centrobolus species together with the relative estimated position of C. silvanus is shown in figure 1. The size of C. silvanus collected in South Africa was calculated as 47.5 mm (n=8). Males size was 43 mm (μ ± σ; n=1) and female size 54 mm (μ ± σ; n=1). An analysis from data presently available showed average SSD for C. silvanus was 1.25581395 differing from 1 (t=1.52753, p=0.085235; n=6) (Table 1).
Discussion
Centrobolus species based on tergite width are clearly recognisable [16,18]. Because SSD was significantly different from 1 in the species, the average size ratio of 1.25581395 for C. silvanus indicates dimorphism. The positive relationship between female and male body sizes in this genus of diplopods is an exception to Rensch’s rule [8-10]. Studies on SSD in other invertebrates have a positive correlation [19]. Figure 1 shows the finding for Centrobolus where the regression of log male size on log female size was significant with a positive slope showing females get larger than males with an increase in body size. The analysis presented here shows SSD based on the horizontal tergite width and size may be a primary factor in mating because the radius of a cylinder can be more powerful in attempts to increase size which is similar to Doratogonus uncinatus where female choice for mating partners is “size selective” [20].
Sexual dimorphism resembles C. inscriptus female width which is positively related to copulation duration and larger females are probably more fecund [21]. Sexual dimorphism in Apfelbeckia insculpta shows female-biased SSD with longer and wider females [11]. On the basis of the findings in C. sagatinus I suggest width is the primary factor and length secondary factor in achieving size differences in Myriapoda [22]. Although no dimorphism was detected in two species of Centrobolus (dubius and lawrencei) differences in dorsal tergite width may occur when larger numbers are studied [23,24].
Conclusion
C. silvanus shows sexual size dimorphism with small males and larger females based on the finding of differences in horizontal tergite width. Sexual dimorphism resembles C. inscriptus and C. sagatinus female width which is positively related to copulation duration and larger females are fecundity selected [18,21].
- Akkari N, Enghoff H (2011) Copulatory-copulatory male succession and male slenderness in Ommatoiulus sempervirilis n. sp., a new insular millipede from Tunisia (Diplopoda: Julida: Julidae). Journal of Zoological Systematics and Evolutionary Research 49:285-291. Link: https://goo.gl/NjtGnq
- Cooper MI (2016) Sexual bimaturism in the millipede Centrobolus inscriptus (Attems). Journal of Entomology and Zoology Studies 4: 86-87. Link: https://goo.gl/MGgmXu
- Cooper MI (2016) Sexual conflict over the duration of copulation in Centrobolus inscriptus (Attems). Journal of Entomology and Zoology Studies 4: 852-854. Link: https://goo.gl/4PxpDV
- Rowe M (2010) Copulation, mating system and sexual dimorphism in an Australian millipede, Cladethosoma clarum. Australian Journal of Zoology 58: 127-132. Link: https://goo.gl/vjqERc
- Telford SR, Dangerfield JM (1993) Mating Tactics in the Tropical Millipede Alloporus uncinatus (Diplopoda: Spirostreptidae). Behaviour 124: 45-56. Link: https://goo.gl/Uh2kRL
- Cooper M (2018) Allometry in Centrobolus. Journal of Entomology and Zoology Studies 6: 284-286.
- Mori E, Mazza G, Lovari S (2017) Sexual Dimorphism. In: Vonk J, Shakelford T (ed.) Encyclopedia of Animal Cognition and Behavior. Switzerland: Springer International Publishing 1-7. Link: https://goo.gl/6yA8cu
- Faiman R, Abergil D, Babocsay G, Razzetti E, Seligman H, et al. (2018) A review of sexual dimorphism of eye size in Colubroidea snakes. Vertebrate Biology 68: 91-108. Link: https://goo.gl/k6XmGm
- Seifan M, Gilad A, Klass K, Werner YL (2009) Ontogenetically stable dimorphism in a lacertid lizard (Acanthodactylus boskianus) with tests of methodology and comments on life-history. Biological Journal of the Linnean Society 97: 275–288. Link: https://goo.gl/GGf61E
- Werner YL, Korolker N, Sion G, Göçmen B (2016) Bergmann’s and Rensch’s rules and the spur-thighed tortoise (Testudo graeca). Biological Journal of the Linnean Society 117: 796–811. Link: https://goo.gl/GtRm8Y
- Ilić BS, Mitić BM, Makarov SE (2017) Sexual dimorphism in Apfelbeckia insculpta (L. Koch, 1867) (Myriapoda: Diplopoda: Callipodida). Archives of Biological Sciences 69: 23-33. Link: https://goo.gl/YA5di8
- Hamer ML (1998) Checklist of Southern African millipedes. Annals of the Natal Museum 39: 11-82. Link: https://goo.gl/dh9yJ2
- Hoffman RL (2001) A note on the status of the name Centrobolus Cook, 1897 Spirobolida, Pachybolidae. Myriapodologica 7: 49-52. Link: https://goo.gl/sXsnJx
- Lawrence RF (1967) The Spiroboloidea (Diplopoda) of the eastern half of southern Africa. Annals of the Natal Museum 18:607-646. Link: https://goo.gl/nwXBUR
- Schubart O. Diplopoda III (1966) South African Animal Life 12: 33-77.
- Cooper MI (2014) Sexual size dimorphism and corroboration of Rensch’s rule in Chersastus millipedes(Diplopoda: Pachybolidae). Journal of Entomology and Zoology Studies 2: 264-266. Link: https://goo.gl/cojy84
- Attems C (1928) The Myriopoda of South Africa. Annals of the South African Museum 26: 305. Link: https://goo.gl/Tj2Y99
- Cooper M (2018) Centrobolus anulatus (Attems, 1934) reversed sexual size dimorphism. Journal of Entomology and Zoology Studies 6: 1569-1572. Link: https://goo.gl/e8is7q
- Webb TJ, Freckleton RP (2007) Only Half Right: Species with Female-Biased Sexual Size Dimorphism Consistently Break Rensch's Rule. PLoS ONE 2: P.e897. Link: https://goo.gl/b5CaMU
- Telford SR, Dangerfield JM (1993) Mating Tactics in the Tropical Millipede Alloporus uncinatus (Diplopoda: Spirostreptidae). Behaviour 124: 45-56. Link: https://goo.gl/ocfMBc
- Cooper MI (2017) The affect of female body width on copulation duration in Centrobolus inscriptus. Journal of Entomology and Zoology Studies 5: 732-733. Link: https://goo.gl/KP1sJa
- Enghoff H, Golovatch S, Short M, Stoev P, Wesener T. Diplopoda - taxonomic overview. In: Minelli A. Treatise on Zoology – Anatomy, Taxonomy, Biology – The Myriapoda 2015; 2:363-453.
- Cooper M (2018) Centrobolus dubius (Schubart, 1966) monomorphism. International Journal of Research Studies in Zoology 4: 17-21. Link:
- Cooper MI (2018) Centrobolus lawrencei (Schubart, 1966) monomorphism. Arthropods 7: Link: https://goo.gl/pjeQHL
- Akkari N, Enghoff H, Metscher BD (2015) A New Dimension in Documenting New Species: High Detail Imaging for Myriapod Taxonomy and First 3D Cybertype of a New Millipede Species (Diplopoda Julida Julidae.) PLoS ONE 10: e0135243. Link: https://goo.gl/6uf199
- Cooper M (2018) Centrobolus sagatinus sexual size dimorphism based on differences in horizontal tergite widths. Journal of Entomology and Zoology Studies 6: 275-277. Link: https://goo.gl/ij8pMY
- Mwabvu T, Lamb J, Slotow R, Hamer M, Barraclough D (2013) Is millipede taxonomy based on gonopod morphology too inclusive? Observations on genetic variation and cryptic speciation in Bicoxidens flavicollis (Diplopoda: Spirostreptida: Spirostreptidae). African Invertebrates 54: 349-356. Link: https://goo.gl/BDKRSA https://goo.gl/Dh62s7
- Pincheira-Donoso D, Hunt J (2015) Fecundity selection theory: concepts and evidence. Biological Reviews 92: 341-356. Link: https://goo.gl/Dh62s7
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
